📖 Lesson: Conservation of Mass
Discover how atoms are rearranged but never created or destroyed in chemical reactions
Introduction & Objectives
Overview of conservation of mass and learning goals
1/3
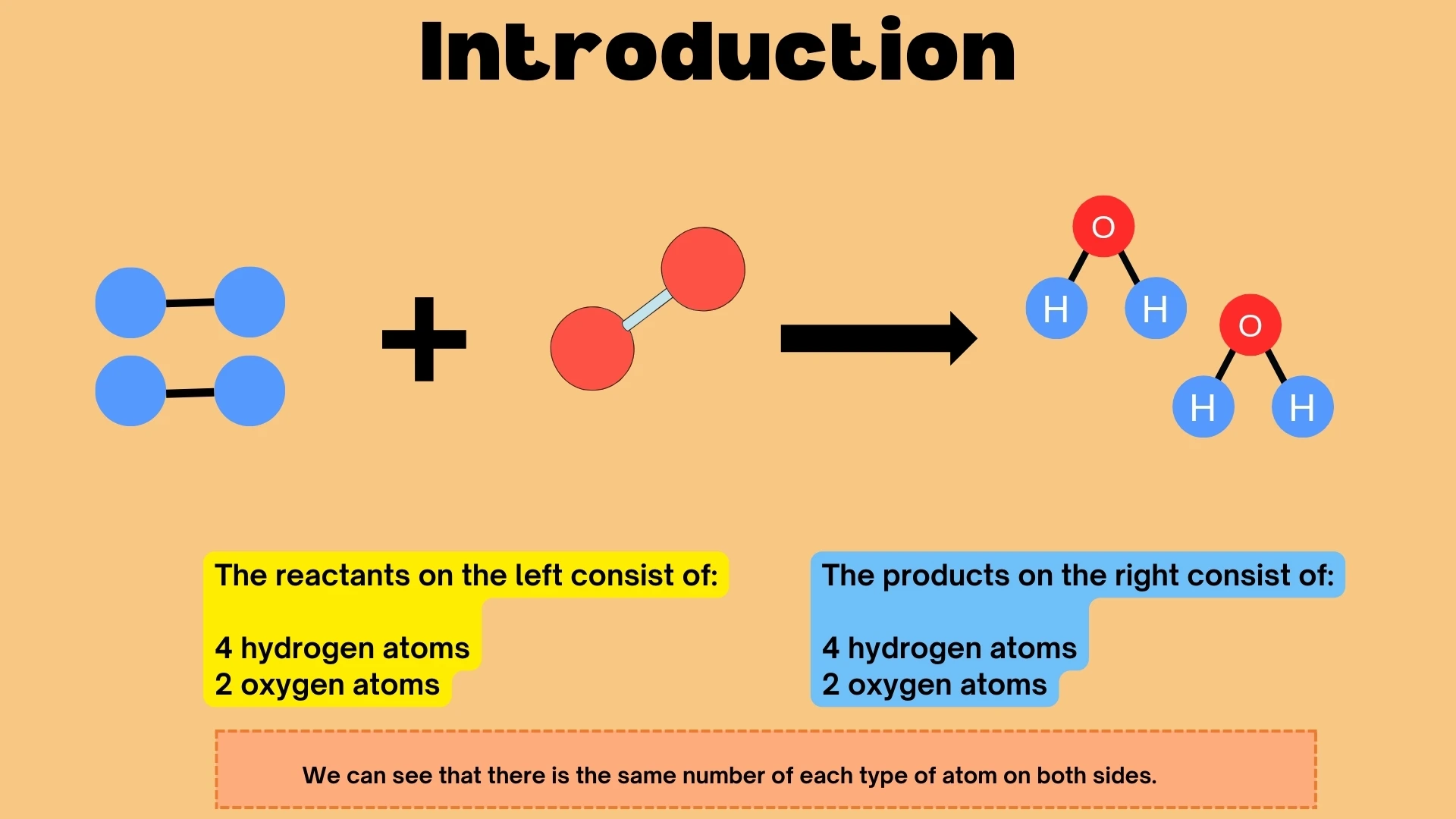
Chemical Reactions & Atoms
How atoms rearrange in chemical reactions
Slide 1/1
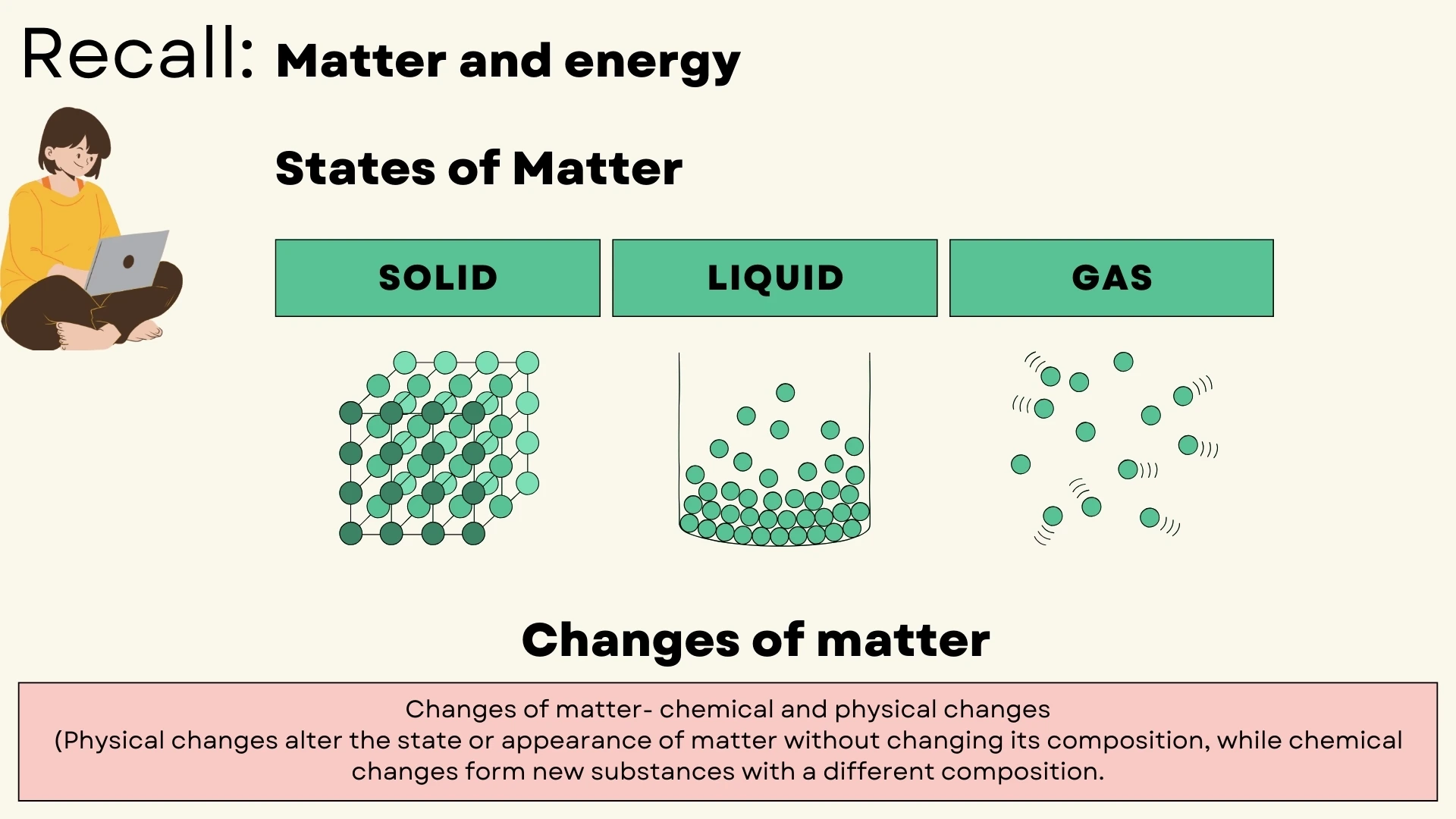
Matter & Changes
States of matter and physical vs chemical changes
1/2
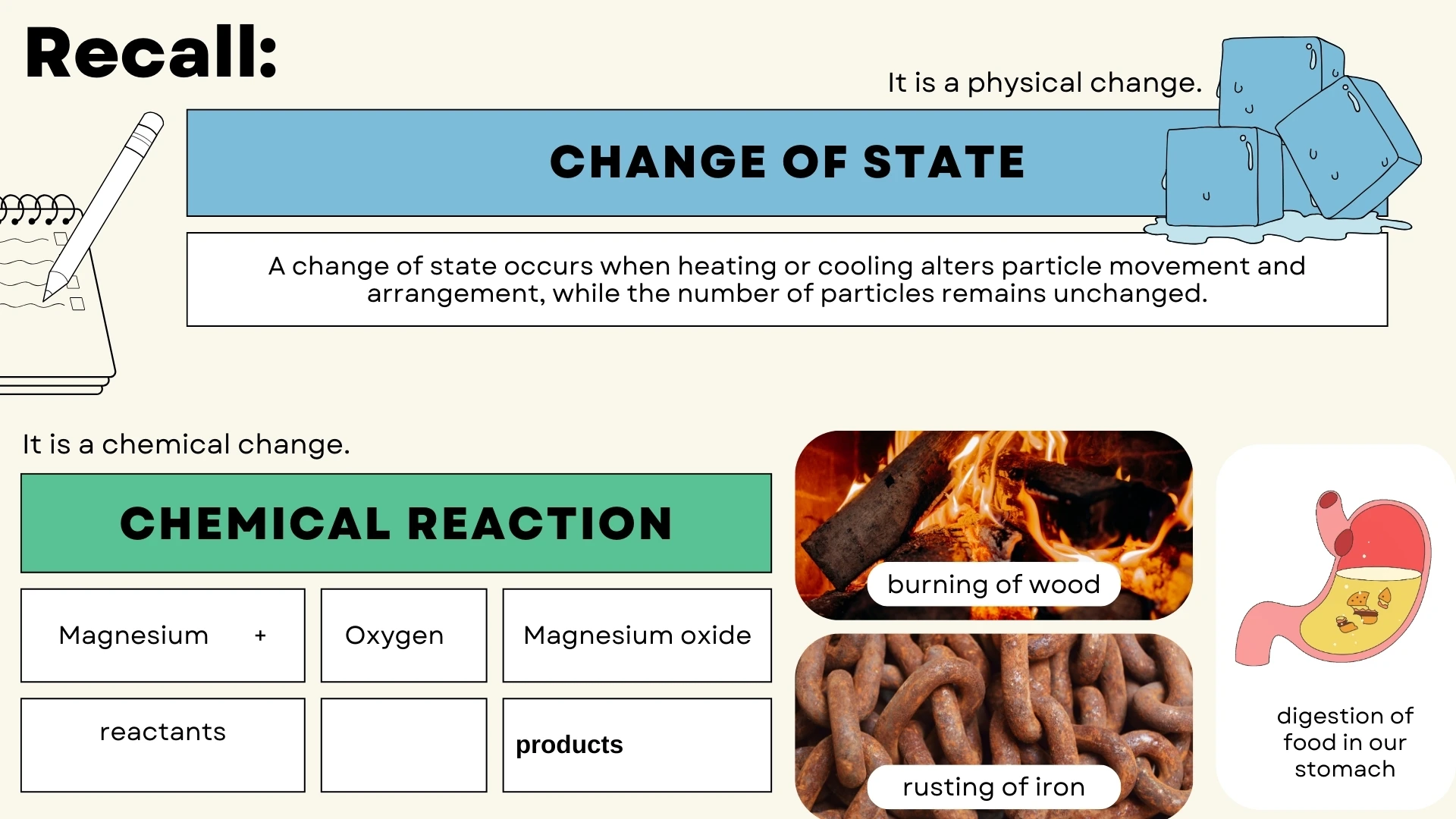
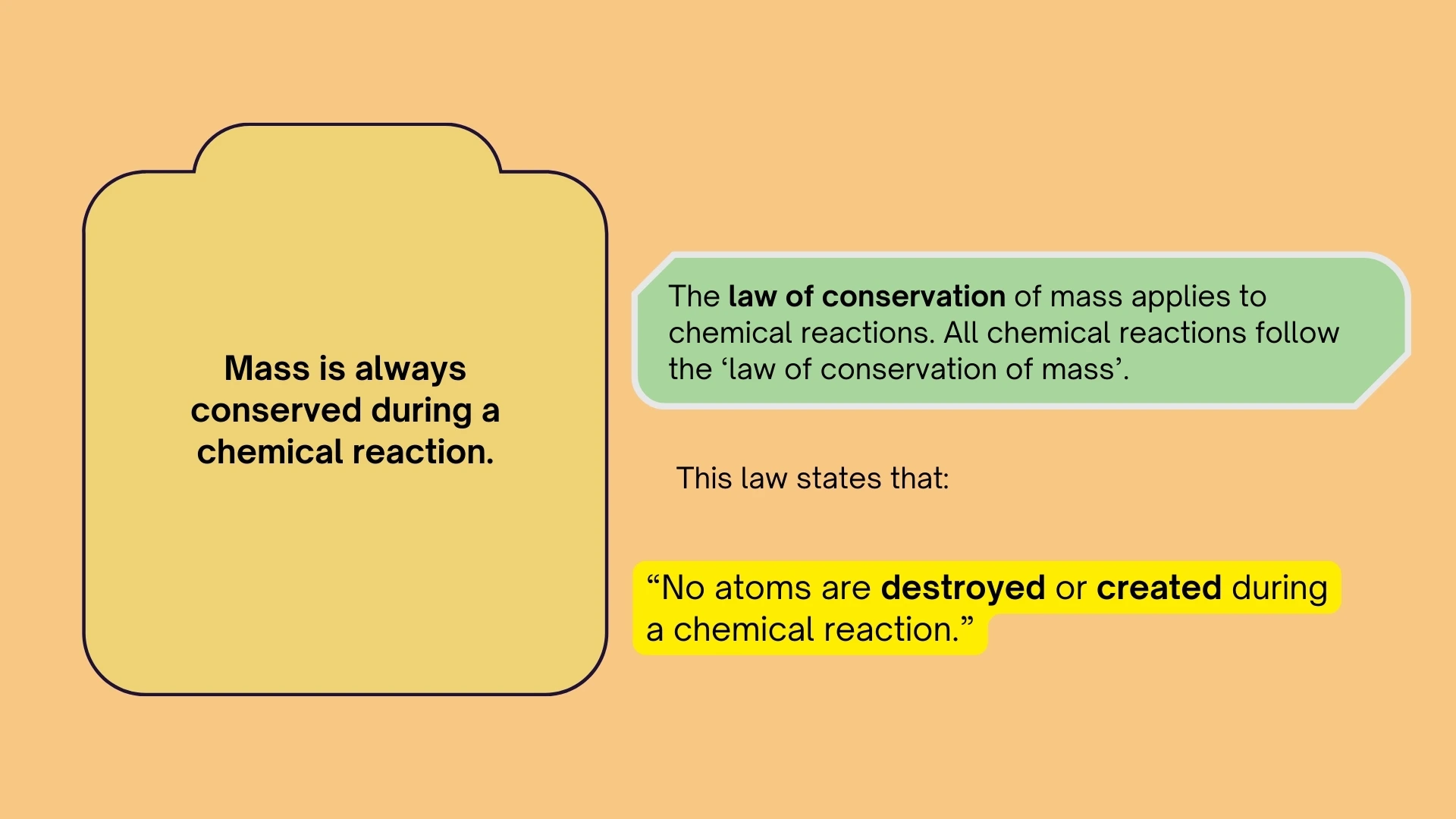
The Law of Conservation of Mass
Mass cannot be created or destroyed in chemical reactions
1/2
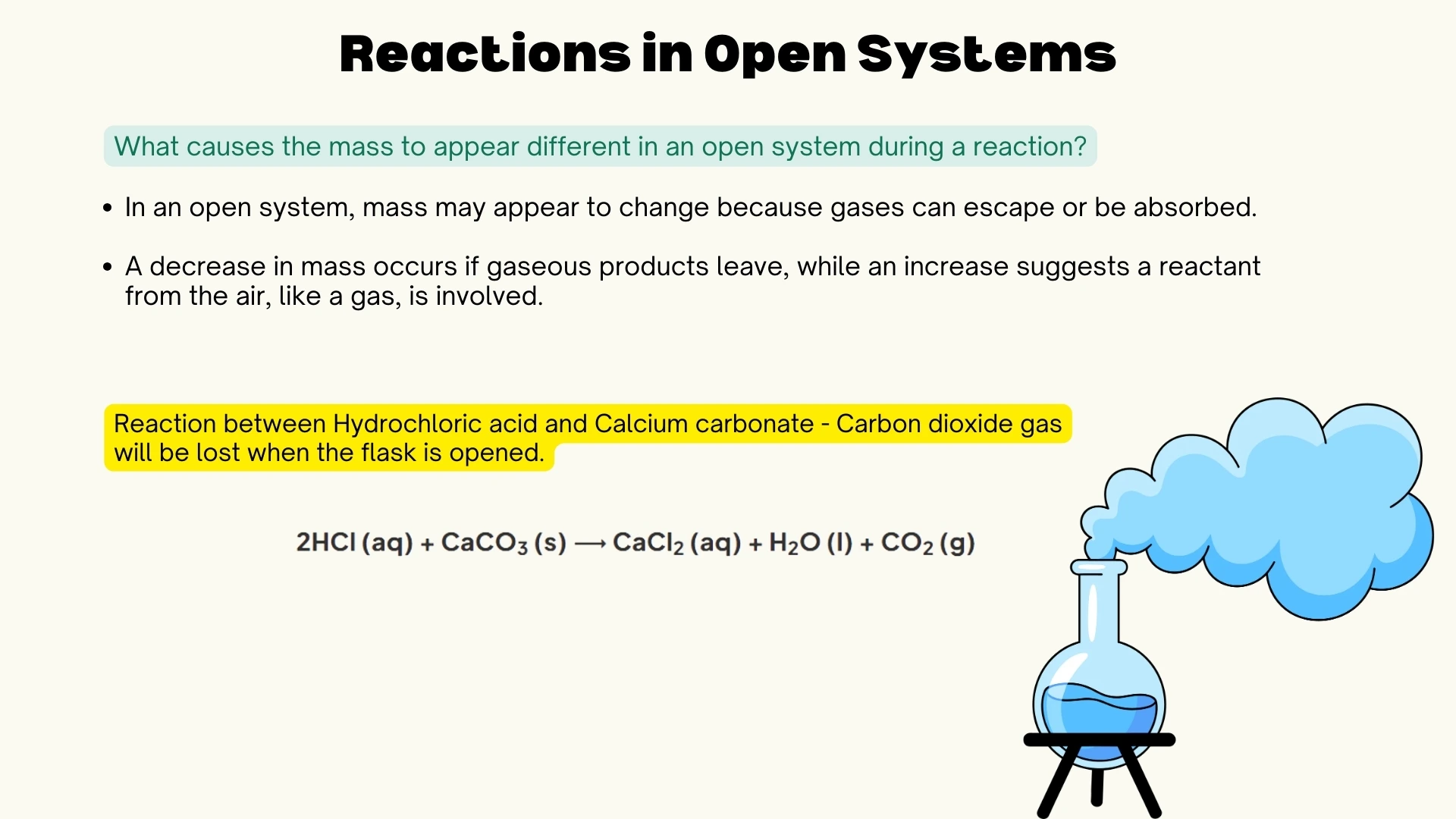
Open vs Closed Systems
Understanding how systems affect mass measurements
1/2
Real Life Applications & Revision
Practical examples and review questions
1/2
🧪 Interactive Activities
Explore conservation of mass through hands-on virtual experiments
Virtual Conservation of Mass Lab
Perform virtual experiments to verify conservation of mass
⚛️
Molecular Level Simulation
Explore chemical reactions at the molecular level! Watch atoms rearrange as reactants become products, and verify that the total number of atoms remains constant throughout the reaction.
Launch Simulation