🧪 Understanding Ions
Positive Ions (Cations)
Metals lose electrons to form positive ions. Their charge matches their group number on the periodic table.
Sodium (Na)
Na⁺
+1Calcium (Ca)
Ca²⁺
+2Aluminium (Al)
Al³⁺
+3Magnesium (Mg)
Mg²⁺
+2Potassium (K)
K⁺
+1Iron(II) (Fe)
Fe²⁺
+2Iron(III) (Fe)
Fe³⁺
+3Copper(I) (Cu)
Cu⁺
+1Copper(II) (Cu)
Cu²⁺
+2Zinc (Zn)
Zn²⁺
+2Silver (Ag)
Ag⁺
+1Ammonium (NH₄)
NH₄⁺
+1Negative Ions (Anions)
Non-metals gain electrons to form negative ions. Their charge is 18 minus their group number.
Chloride (Cl)
Cl⁻
-1Oxide (O)
O²⁻
-2Sulfate (SO₄)
SO₄²⁻
-2Nitrate (NO₃)
NO₃⁻
-1Carbonate (CO₃)
CO₃²⁻
-2Hydroxide (OH)
OH⁻
-1Phosphate (PO₄)
PO₄³⁻
-3Dichromate (Cr₂O₇)
Cr₂O₇²⁻
-2📝 Interactive Worksheets
Practice Chemical Formula Writing
Click on any worksheet to view it in full size. Work through the exercises to master chemical formula writing!

Exercise 1: Formula Writing
Write chemical formulas for ionic compounds
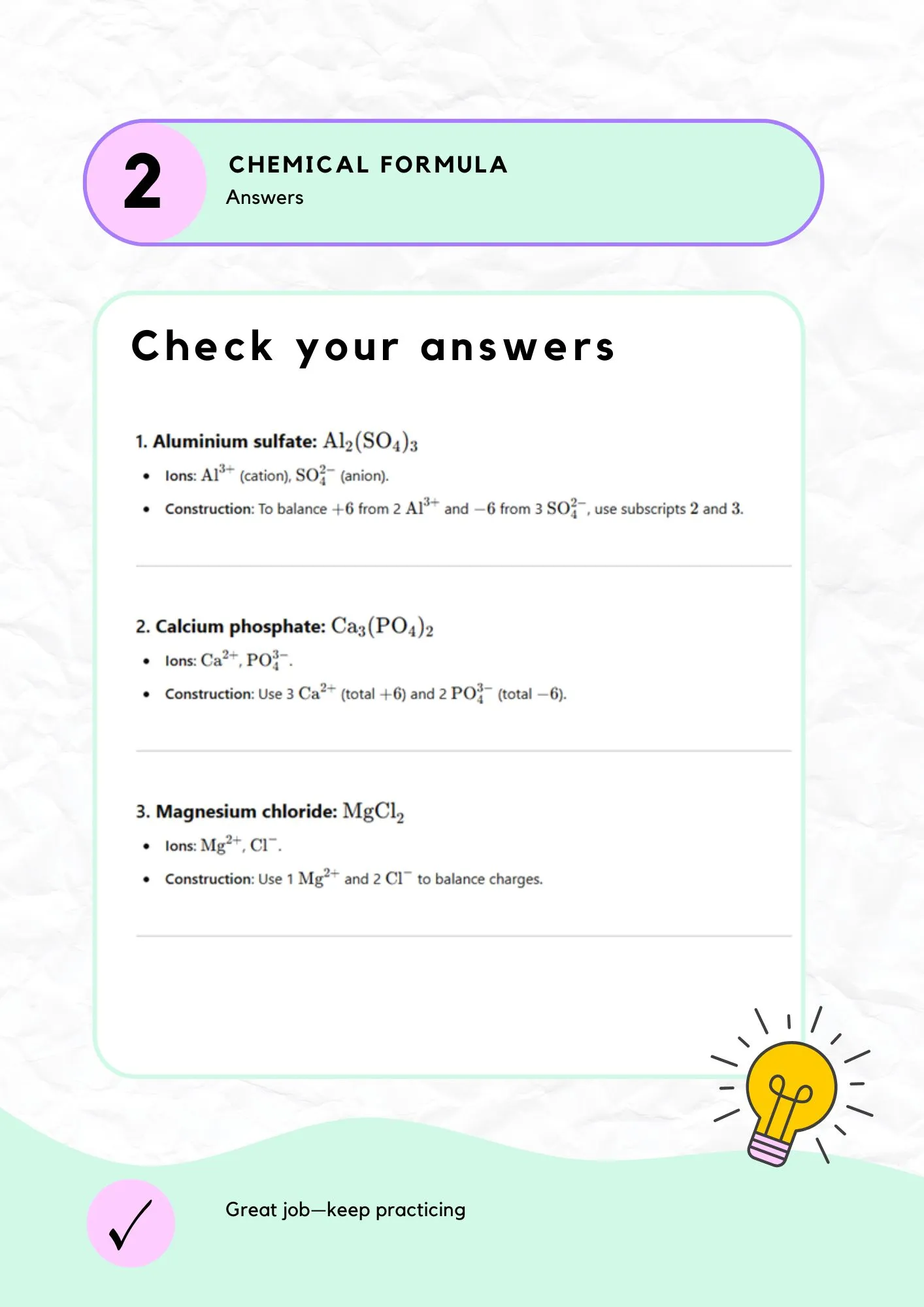
Exercise 1: Answers
Check your answers with detailed explanations
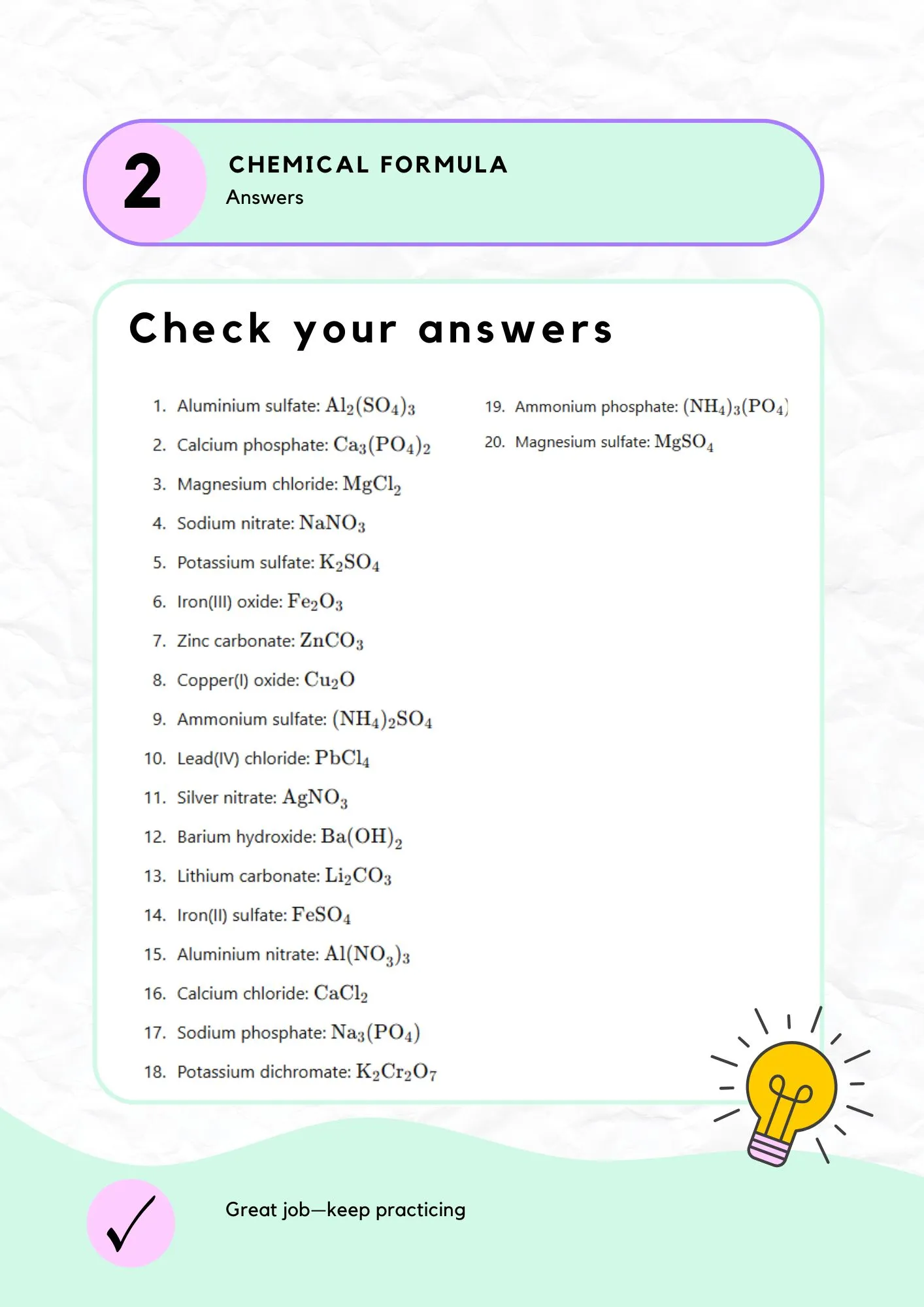
Exercise 1: Full Answer Key
Complete list of all formula answers
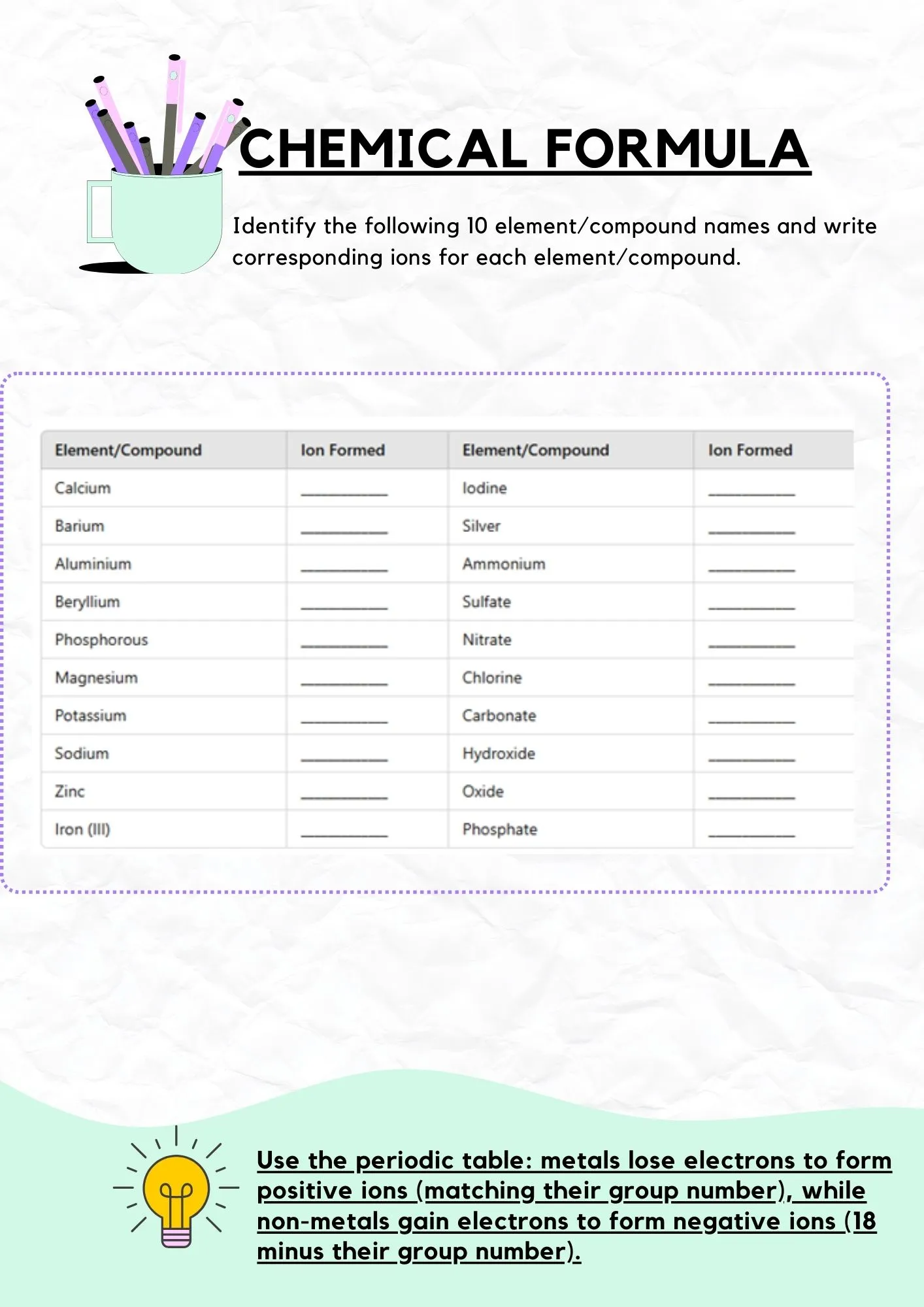
Exercise 2: Ion Formation
Identify ions formed by elements and compounds
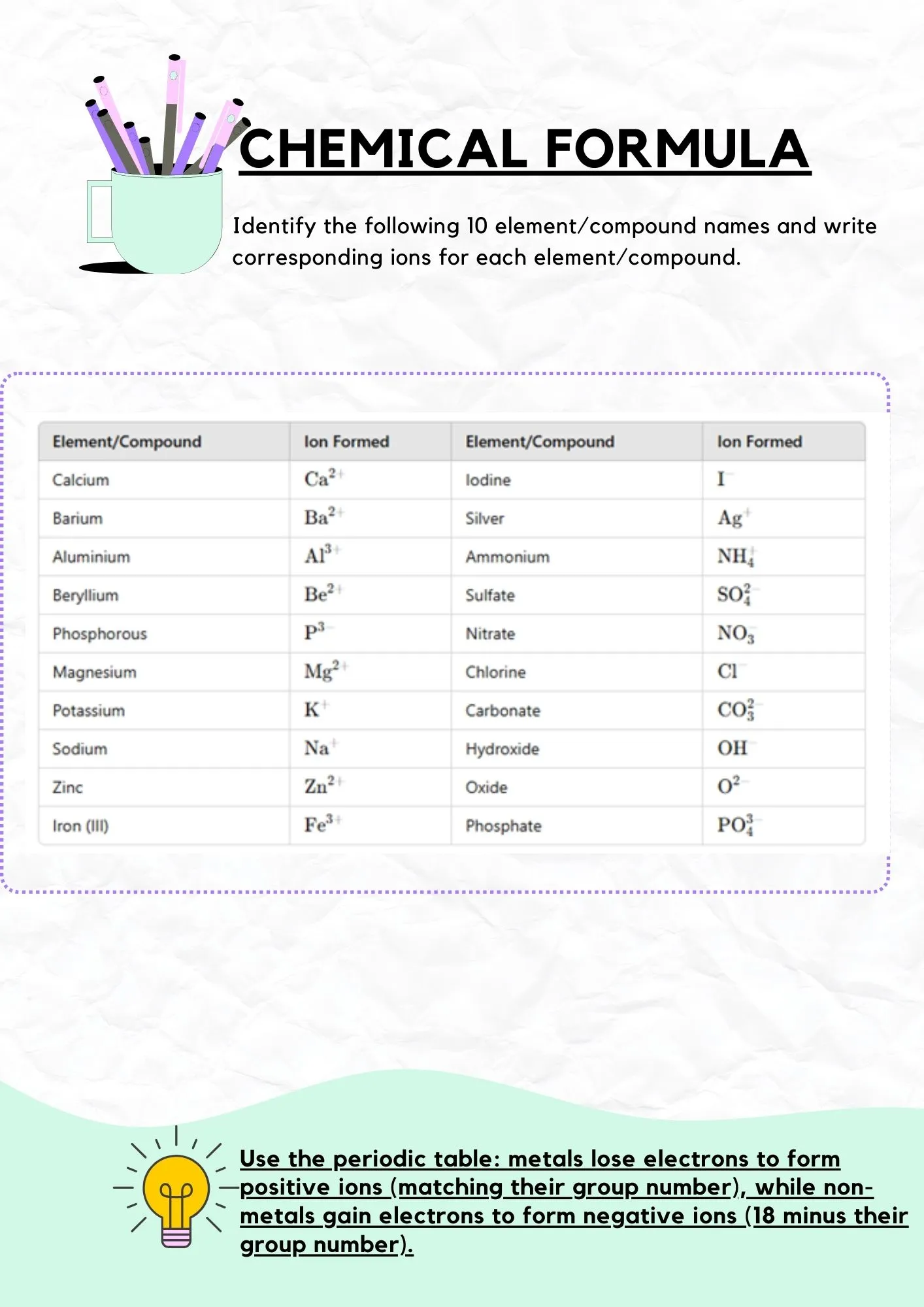
Exercise 2: Answers
Check your ion formation answers
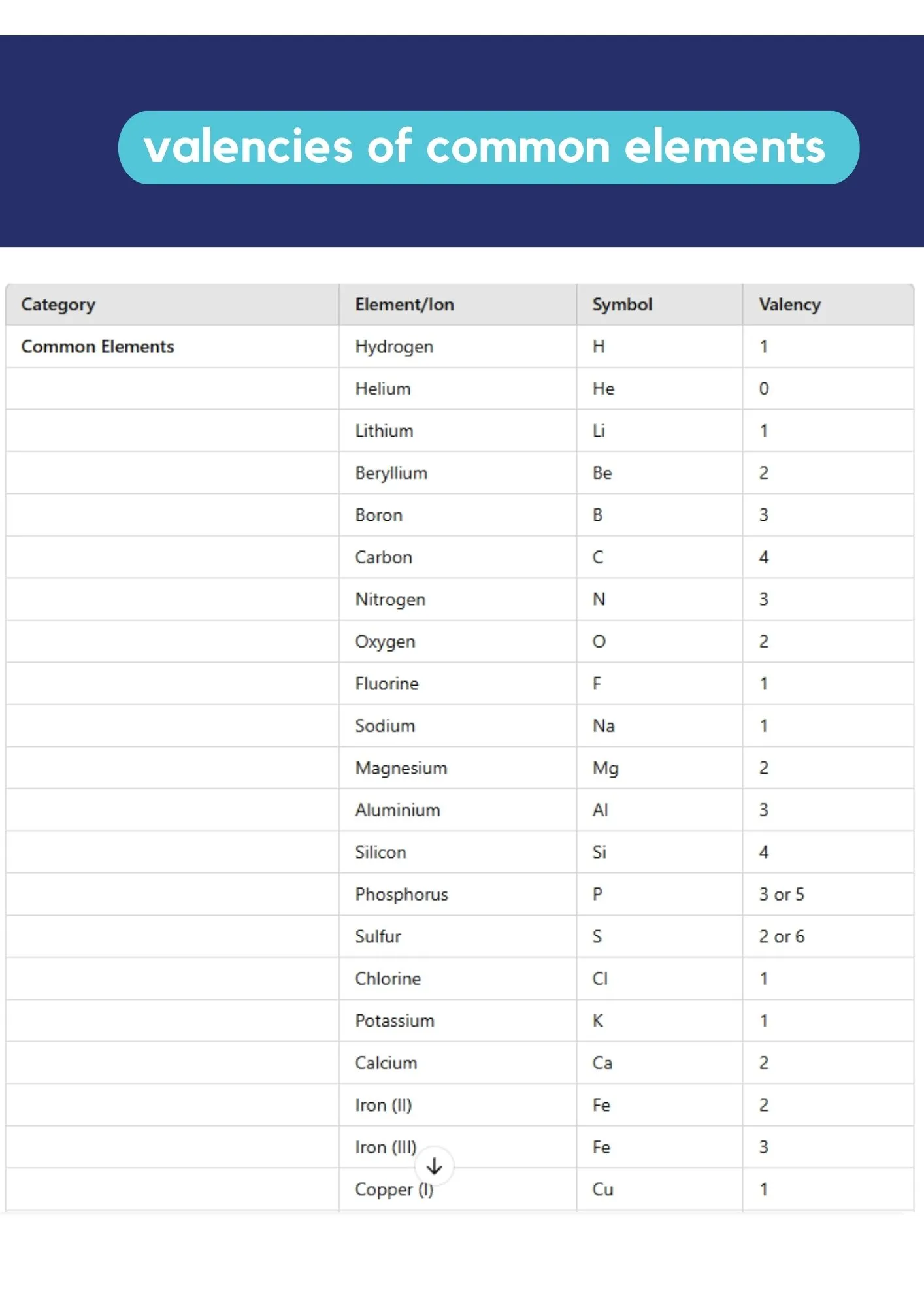
Reference: Valencies of Elements
Quick reference for common element valencies
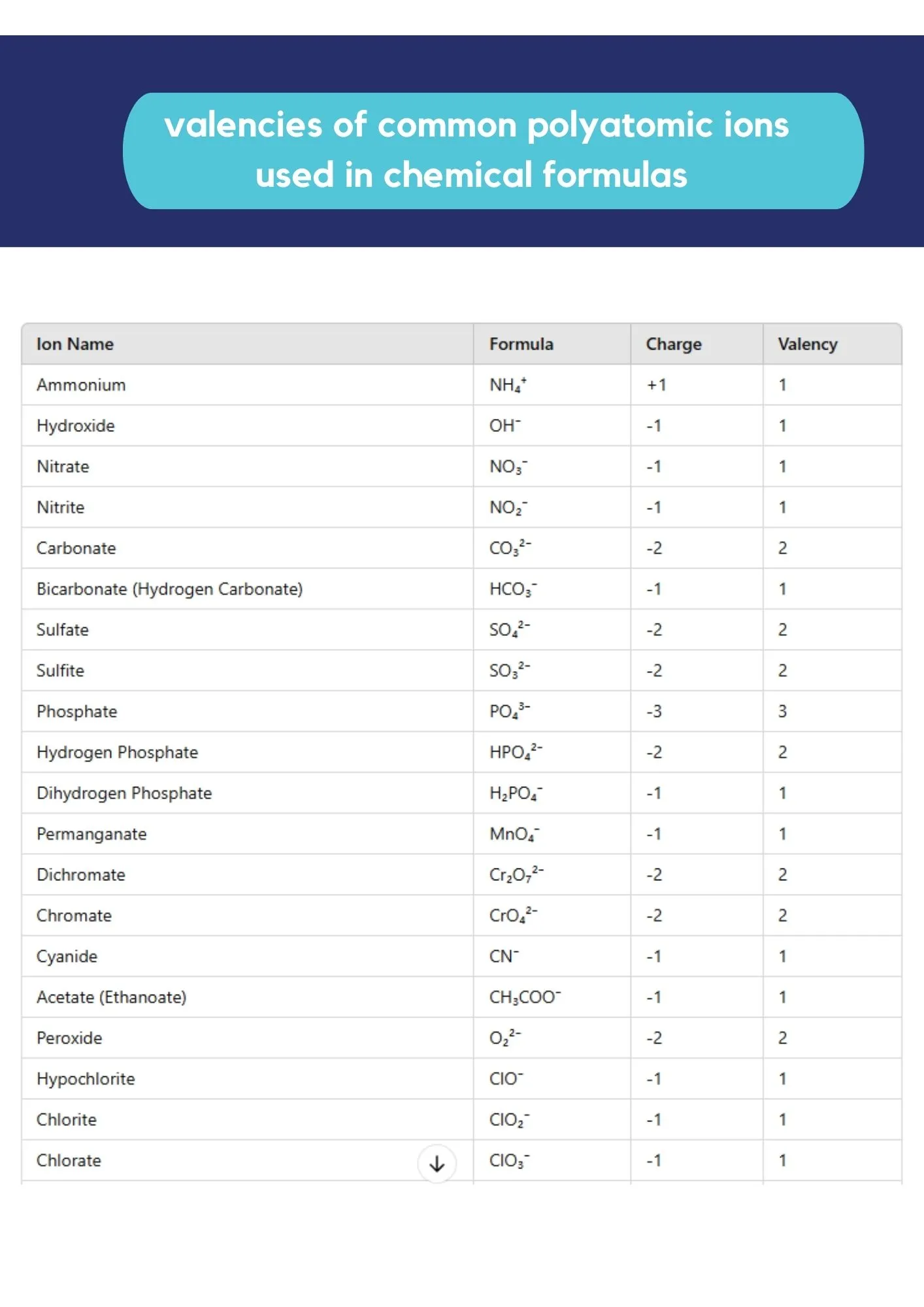
Reference: Polyatomic Ions
Valencies of common polyatomic ions
🎮 Interactive Activities
Coming Soon: Learning Activities
Exciting interactive activities are on the way to help you master chemical formulas!
Formula Builder Game
Build chemical formulas by dragging and dropping ions. Get instant feedback on your answers!
Coming SoonFormula Quiz Challenge
Test your knowledge with timed quizzes and compete with your classmates!
Coming Soon