📖 Lesson: Atomic Structure
Explore atoms, subatomic particles, and how electrons are arranged
🎮 Interactive Activities
Test your knowledge with these fun activities
🧠 Memory Match Game
Match the terms with their definitions!
✏️ Fill in the Blanks
Click on a word from the word bank, then click on the blank to fill it
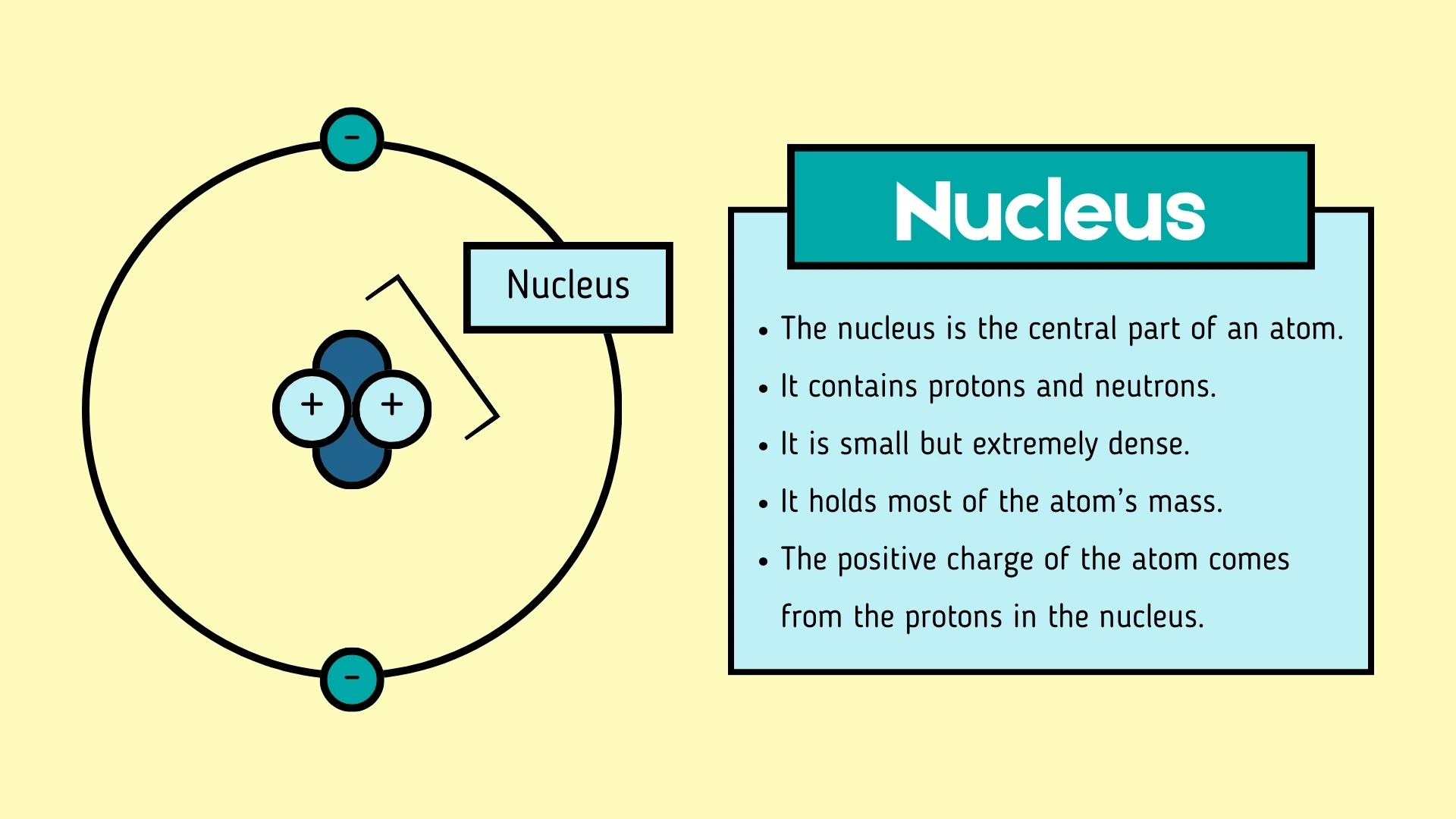
1. The central part of an atom is called the , which contains protons and neutrons.
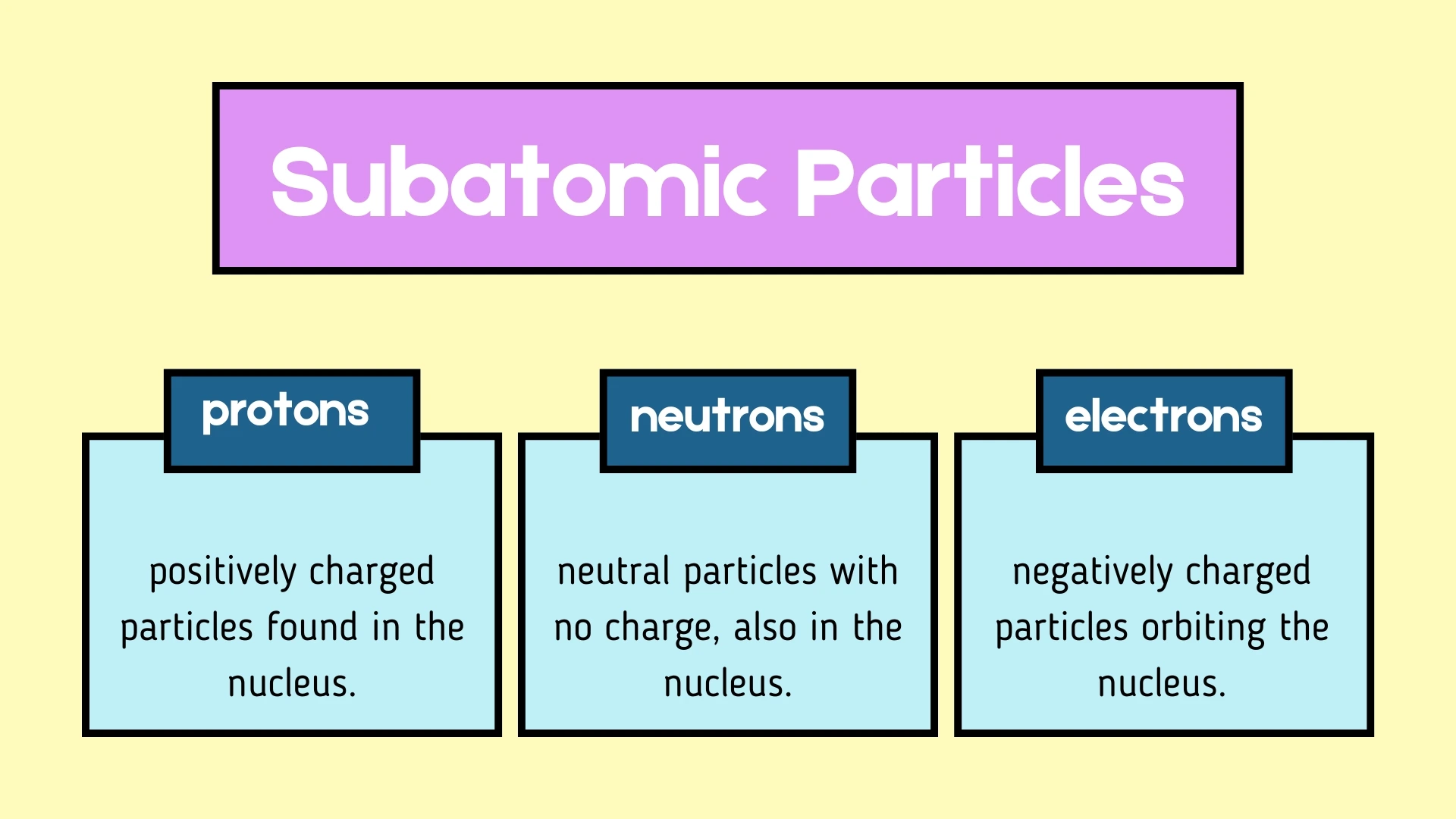
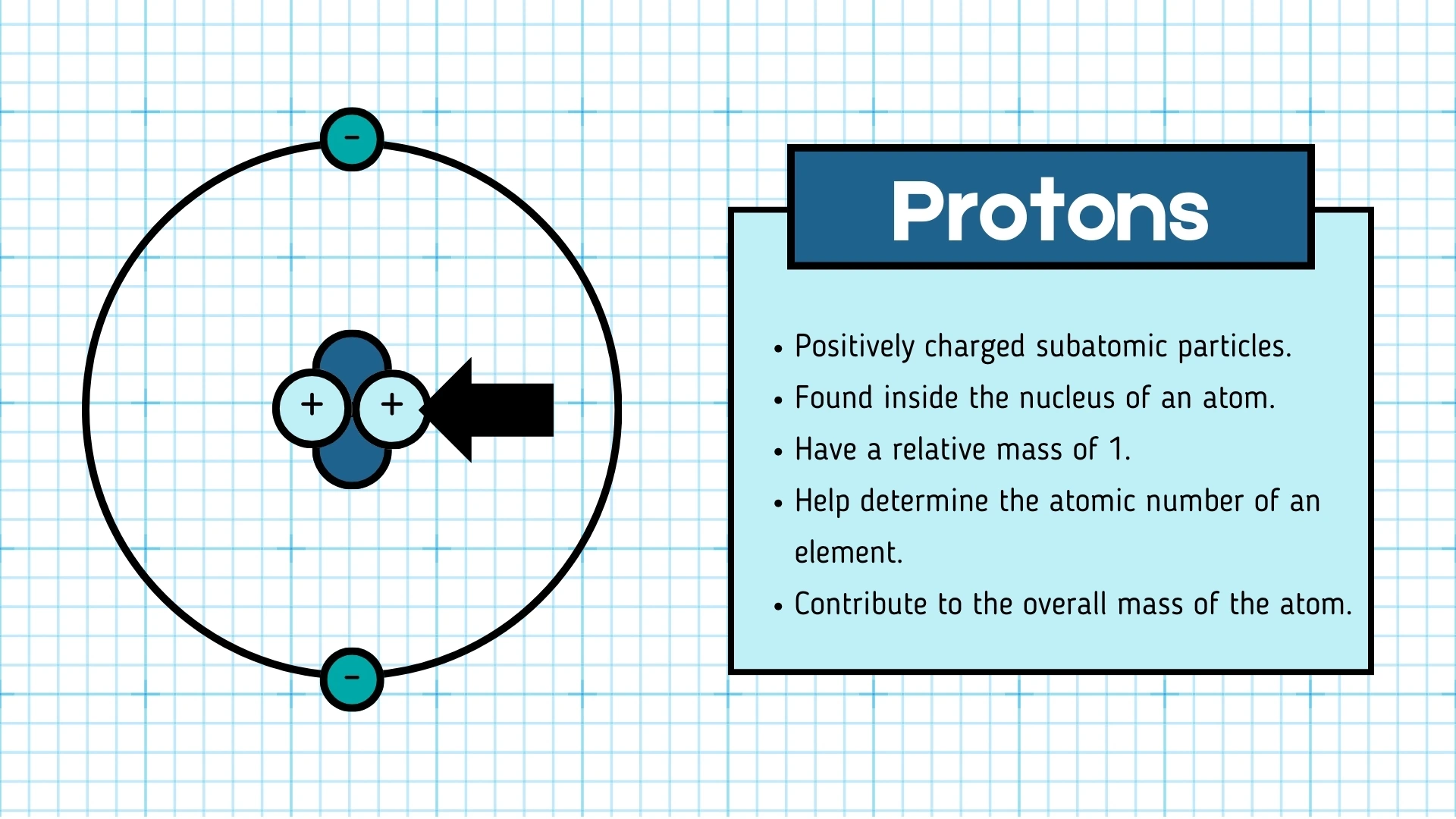
2. are positively charged particles found in the nucleus.
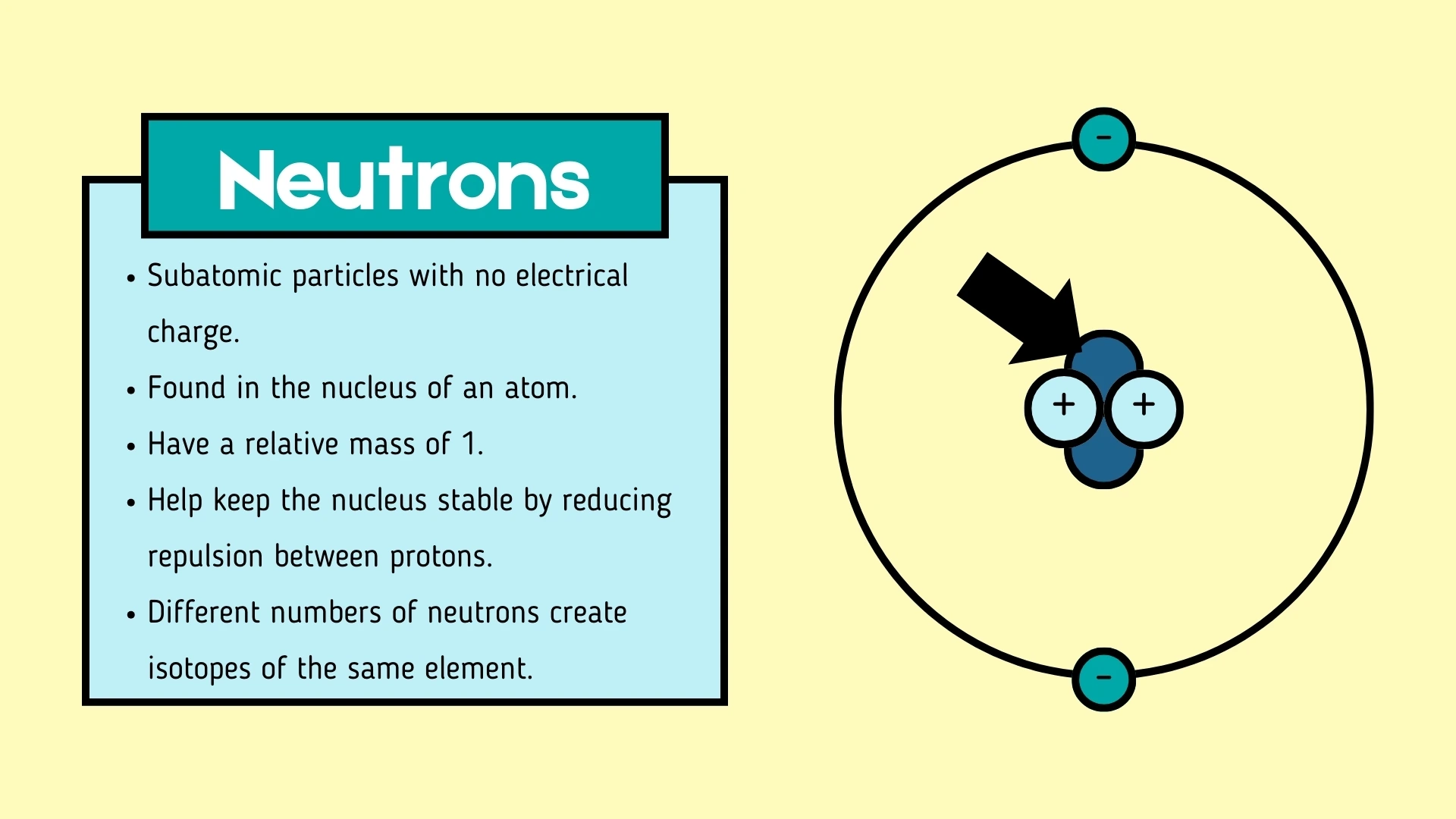
3. have no electrical charge and are also found in the nucleus.
4. are negatively charged particles that orbit around the nucleus.
5. Protons have a charge while electrons have a charge.
6. Atoms are because they have equal numbers of protons and electrons.
7. Electrons are arranged in energy levels, also called .
You got 0/8 correct!
📚 Flashcards
Click on each card to reveal the definition
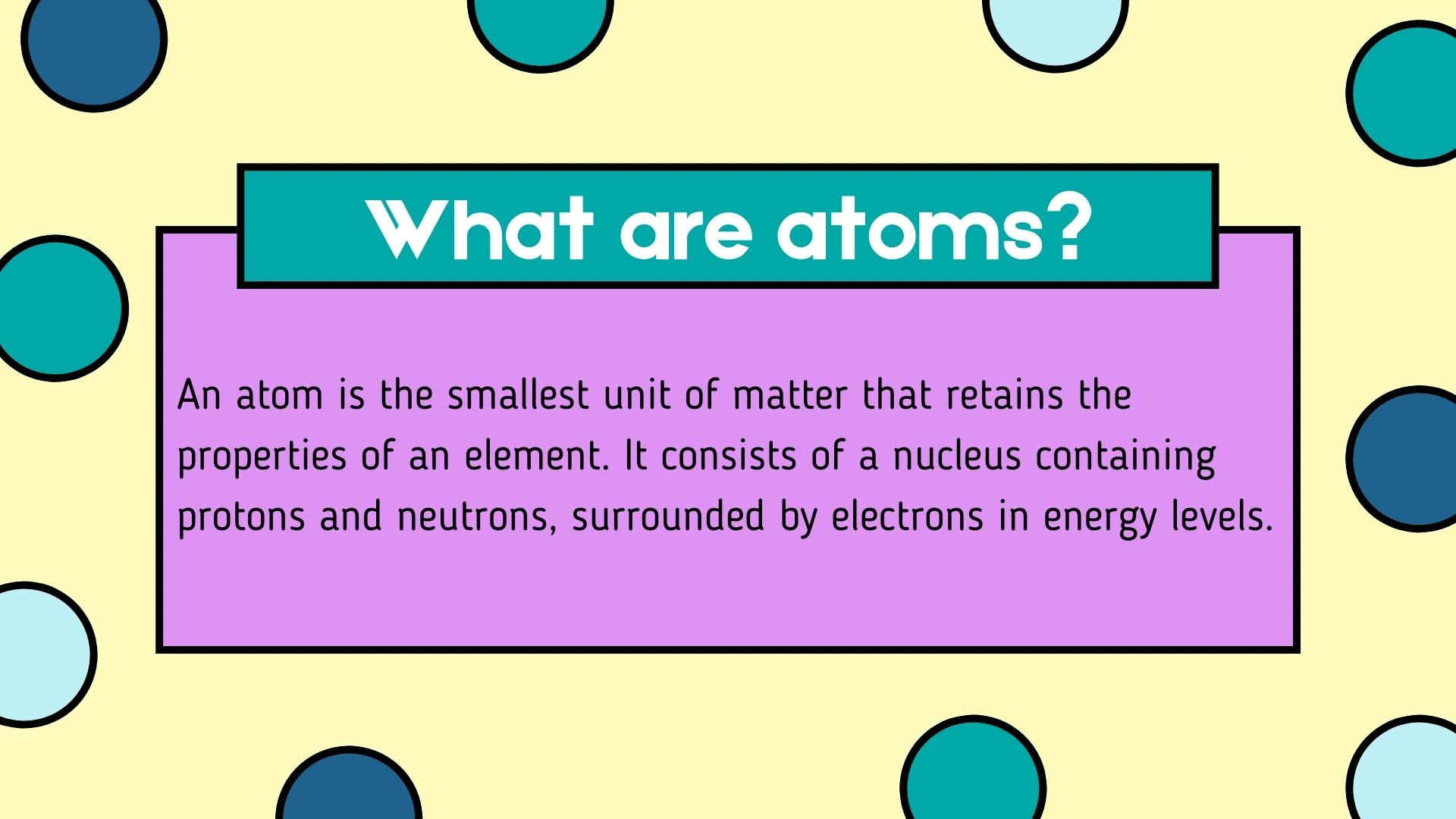
Atom
Click to revealThe smallest unit of matter that retains the properties of an element. It consists of a nucleus containing protons and neutrons, surrounded by electrons in energy levels.
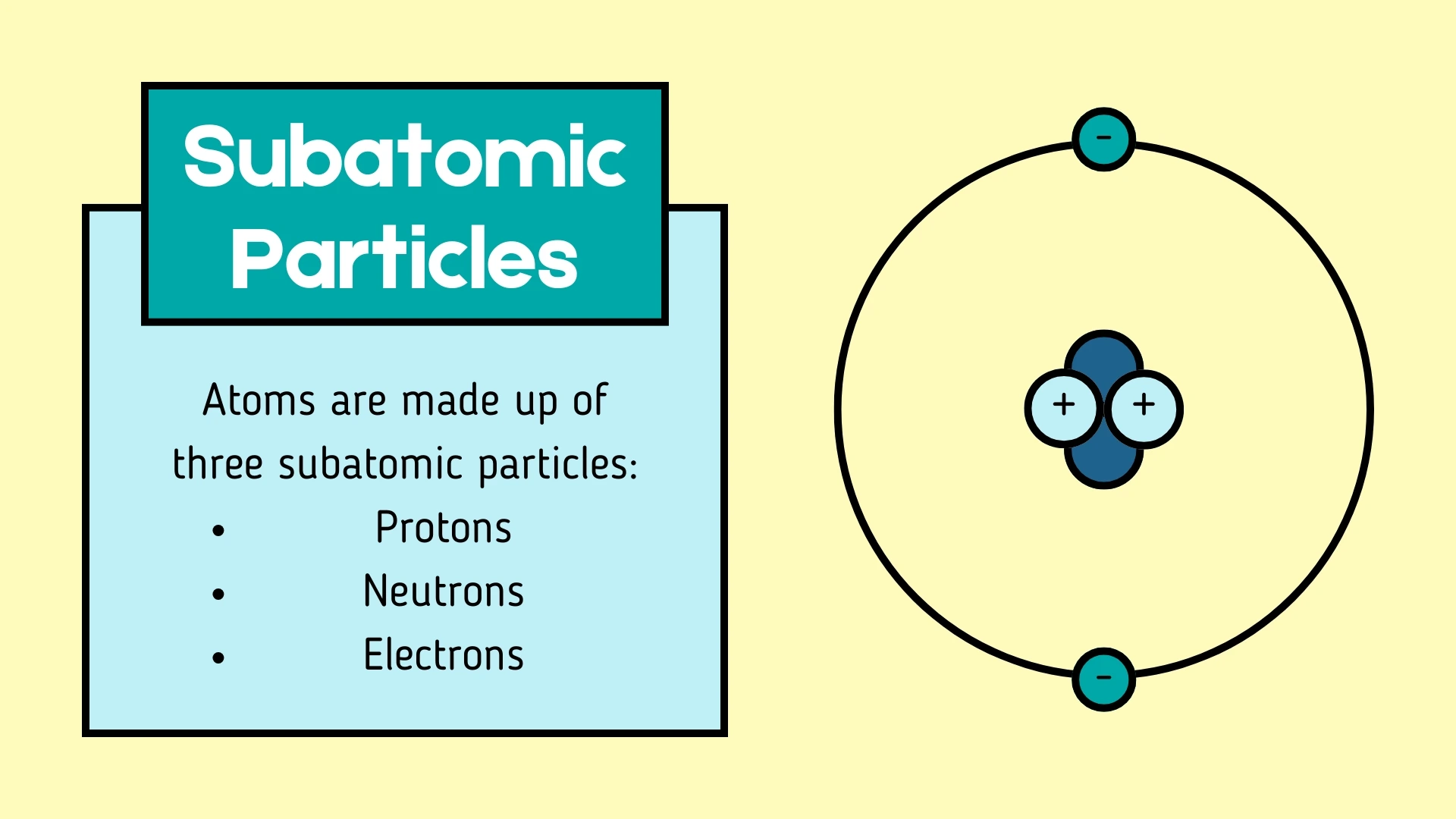
Proton
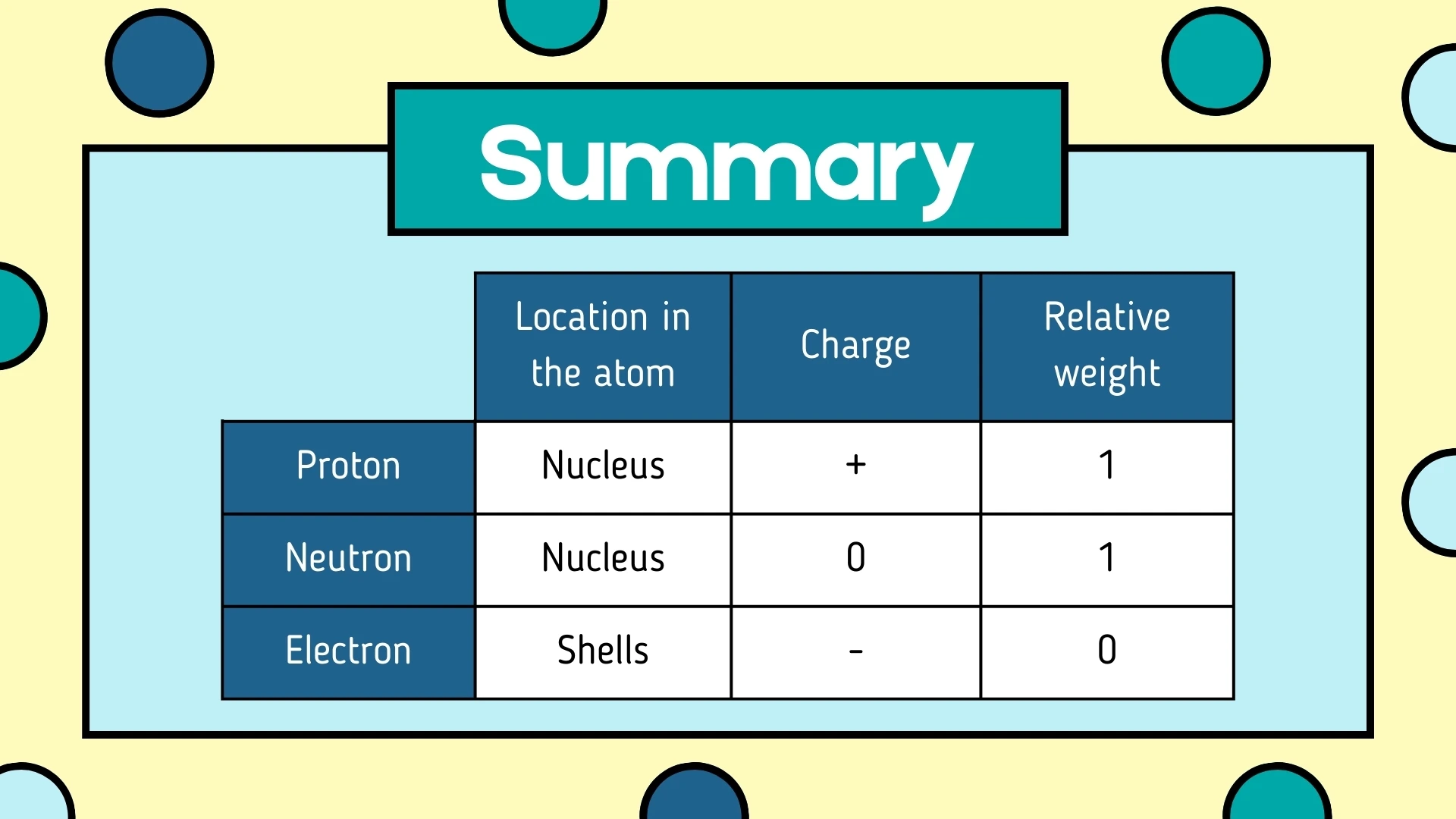
Click to revealA positively charged subatomic particle found in the nucleus. It has a relative mass of 1 and determines the atomic number of an element.
Neutron
Click to revealA subatomic particle with no electrical charge (neutral) found in the nucleus. It has a relative mass of 1 and helps keep the nucleus stable.
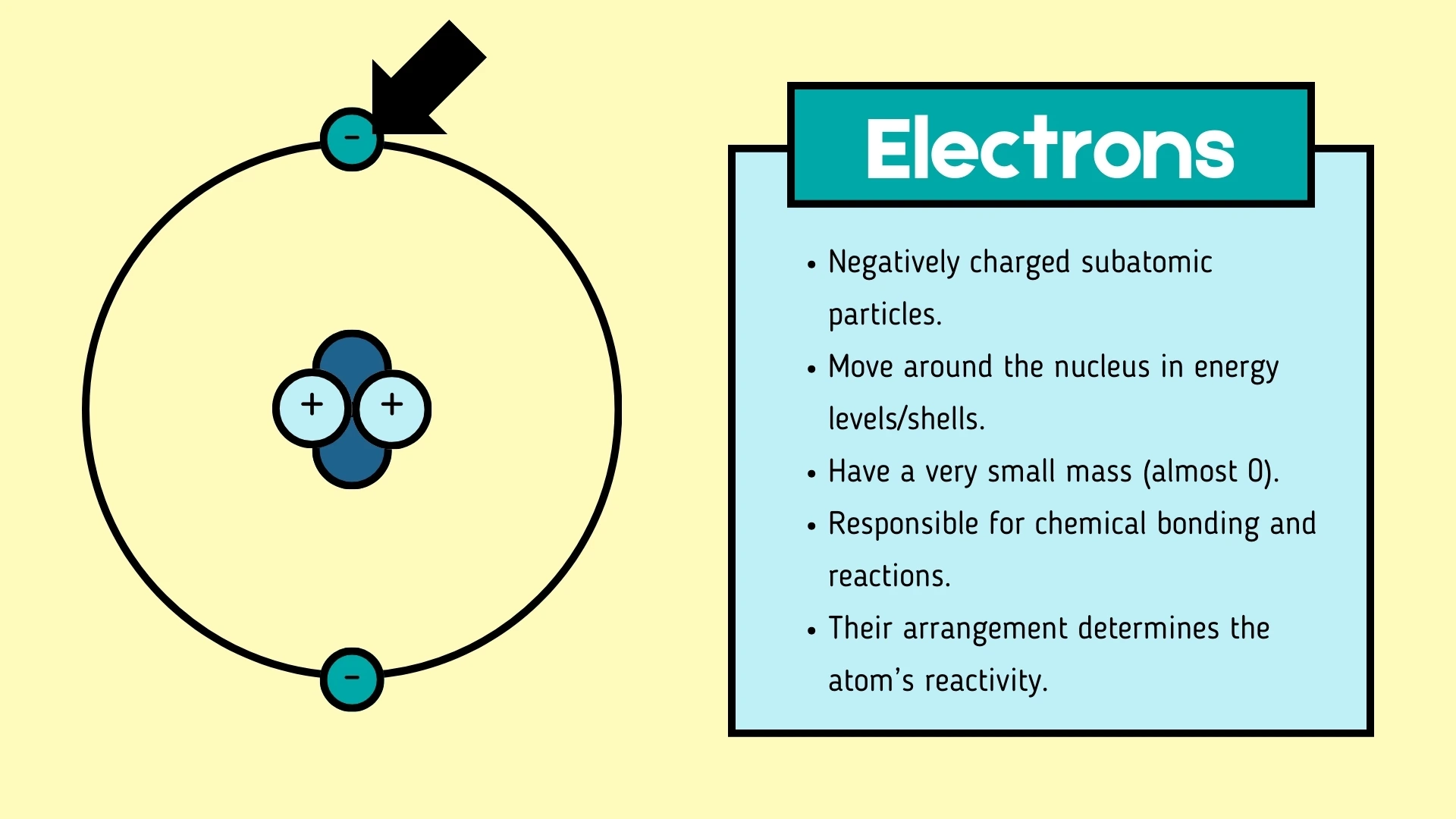
Electron
Click to revealA negatively charged subatomic particle that orbits the nucleus in energy levels (shells). It has a very small mass (almost 0) and is responsible for chemical bonding.
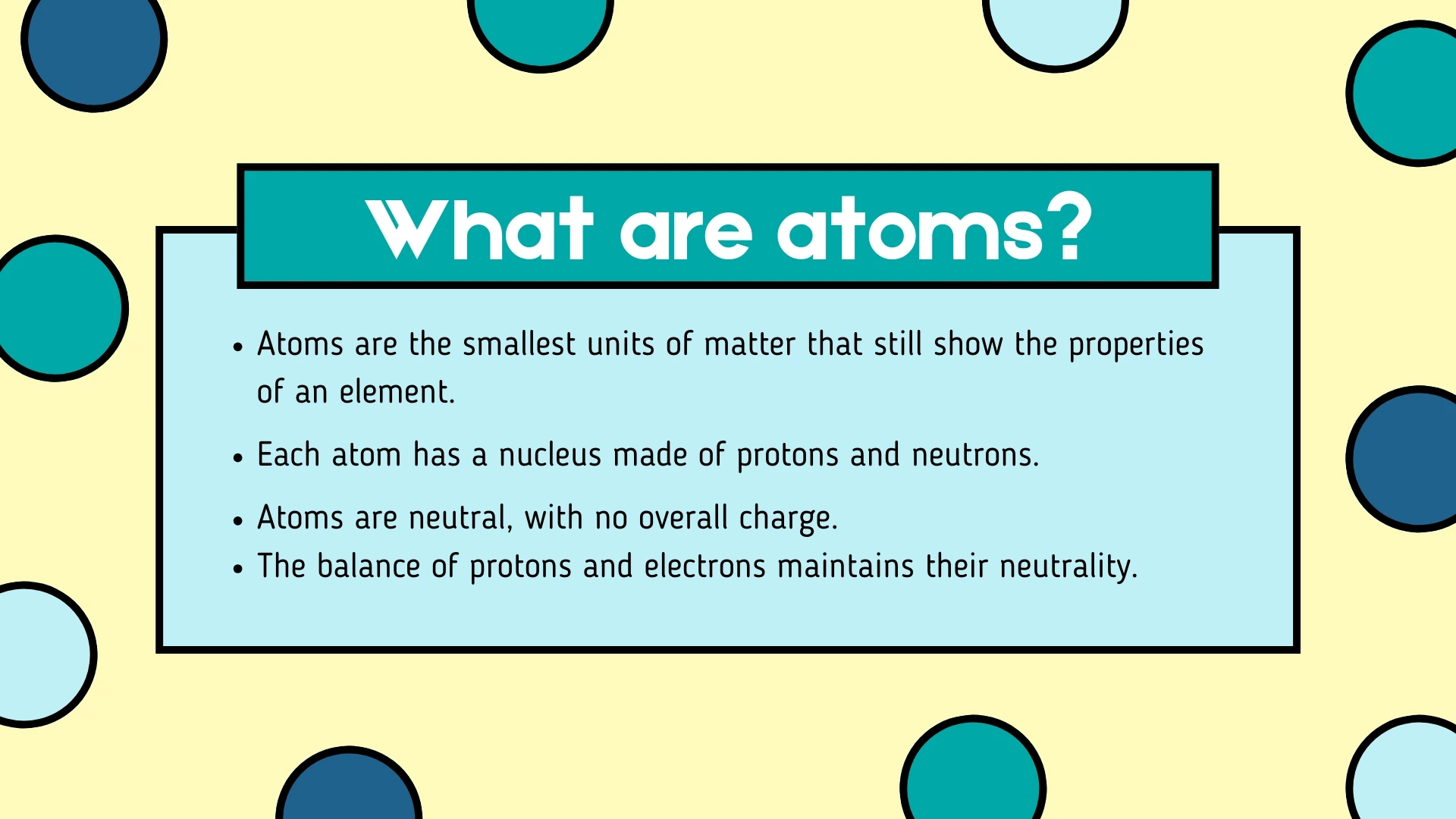
Nucleus
Click to revealThe central part of an atom containing protons and neutrons. It is small but extremely dense and holds most of the atom's mass.
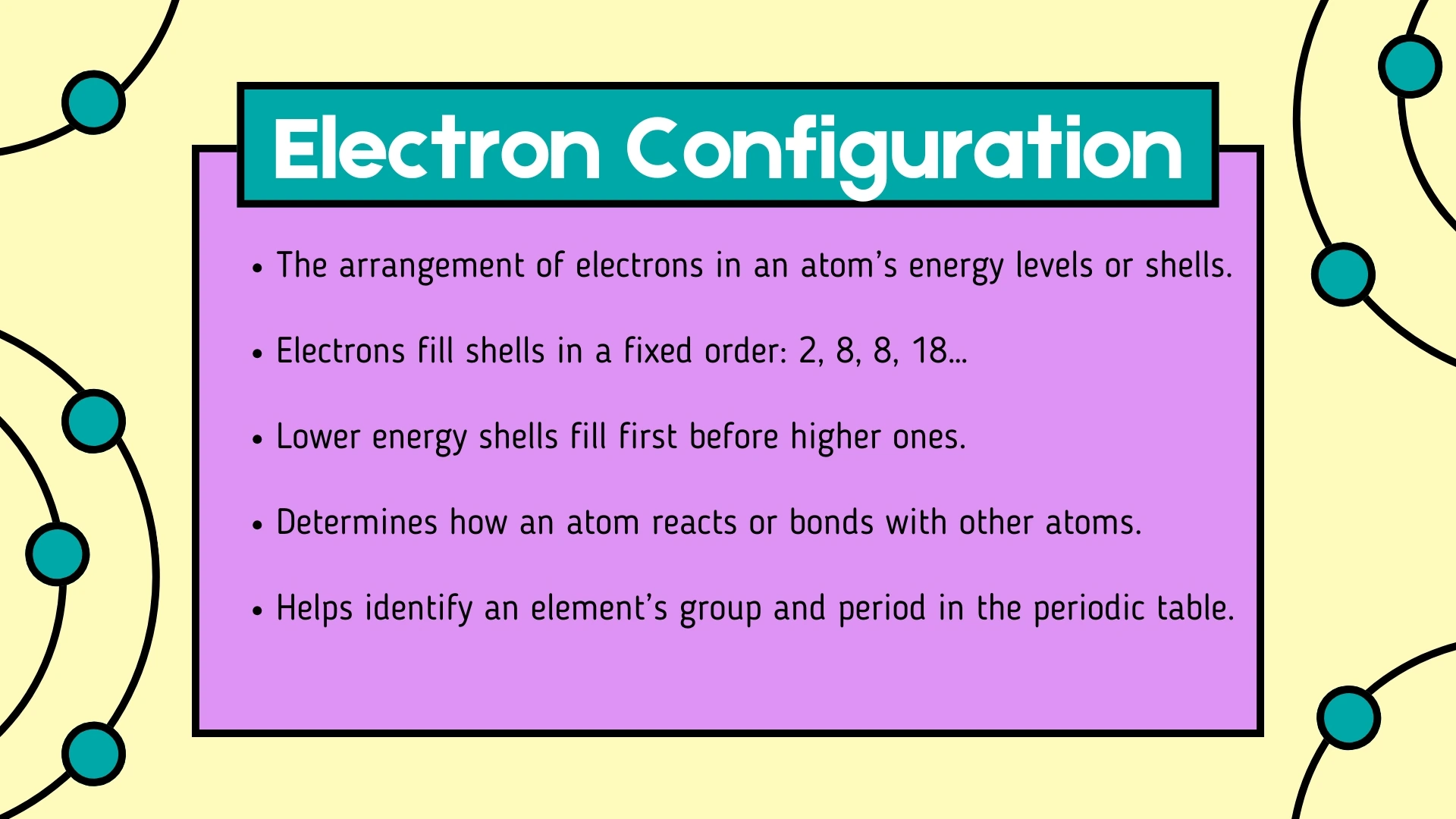
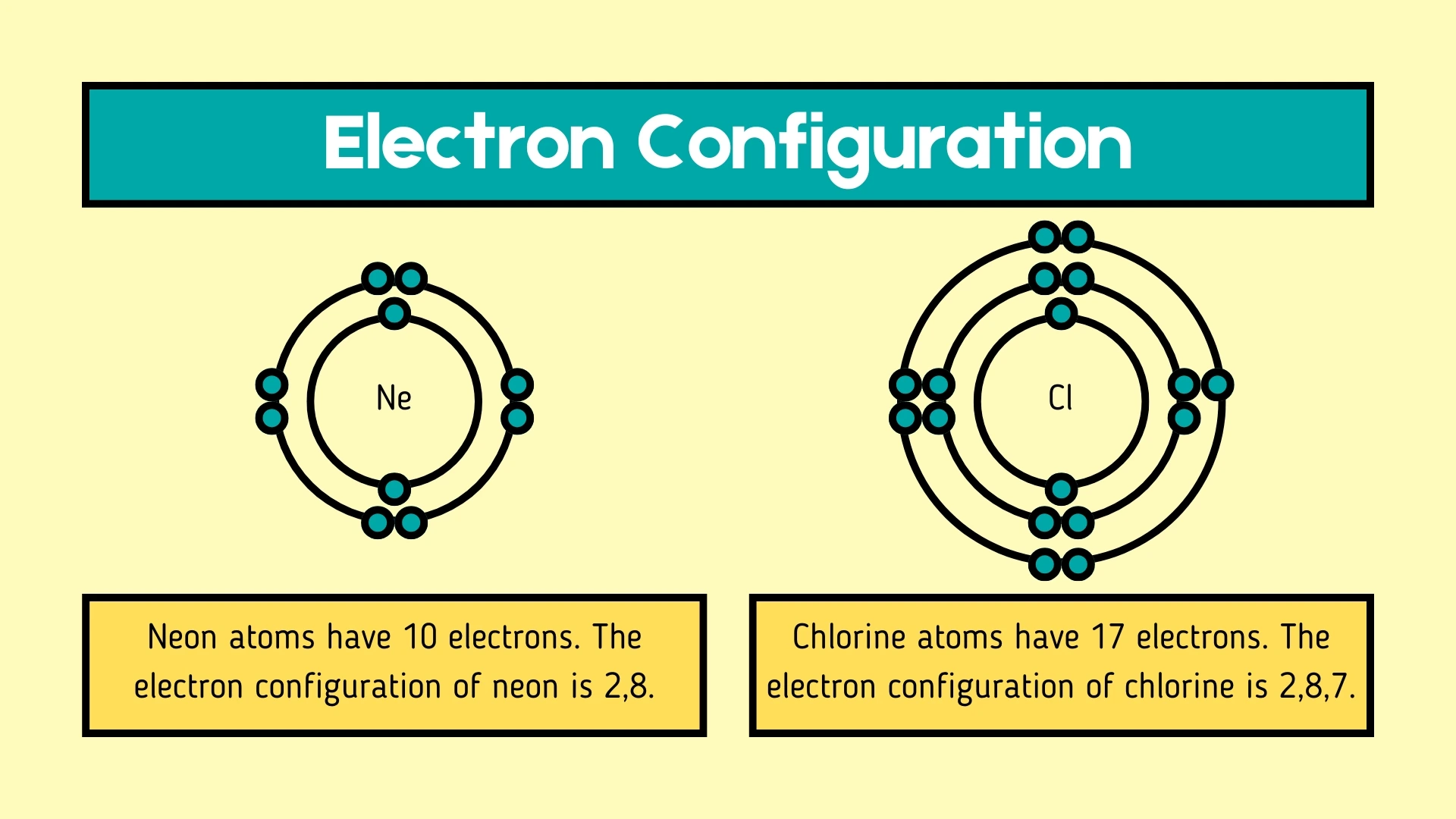
Electron Configuration
Click to revealThe arrangement of electrons in an atom's energy levels or shells. Electrons fill shells in order: 2, 8, 8, 18... with lower shells filling first.
Atomic Number
Click to revealThe number of protons in the nucleus of an atom. It determines what element the atom is and equals the number of electrons in a neutral atom.
Mass Number
Click to revealThe total number of protons and neutrons in the nucleus of an atom. Mass Number = Protons + Neutrons.
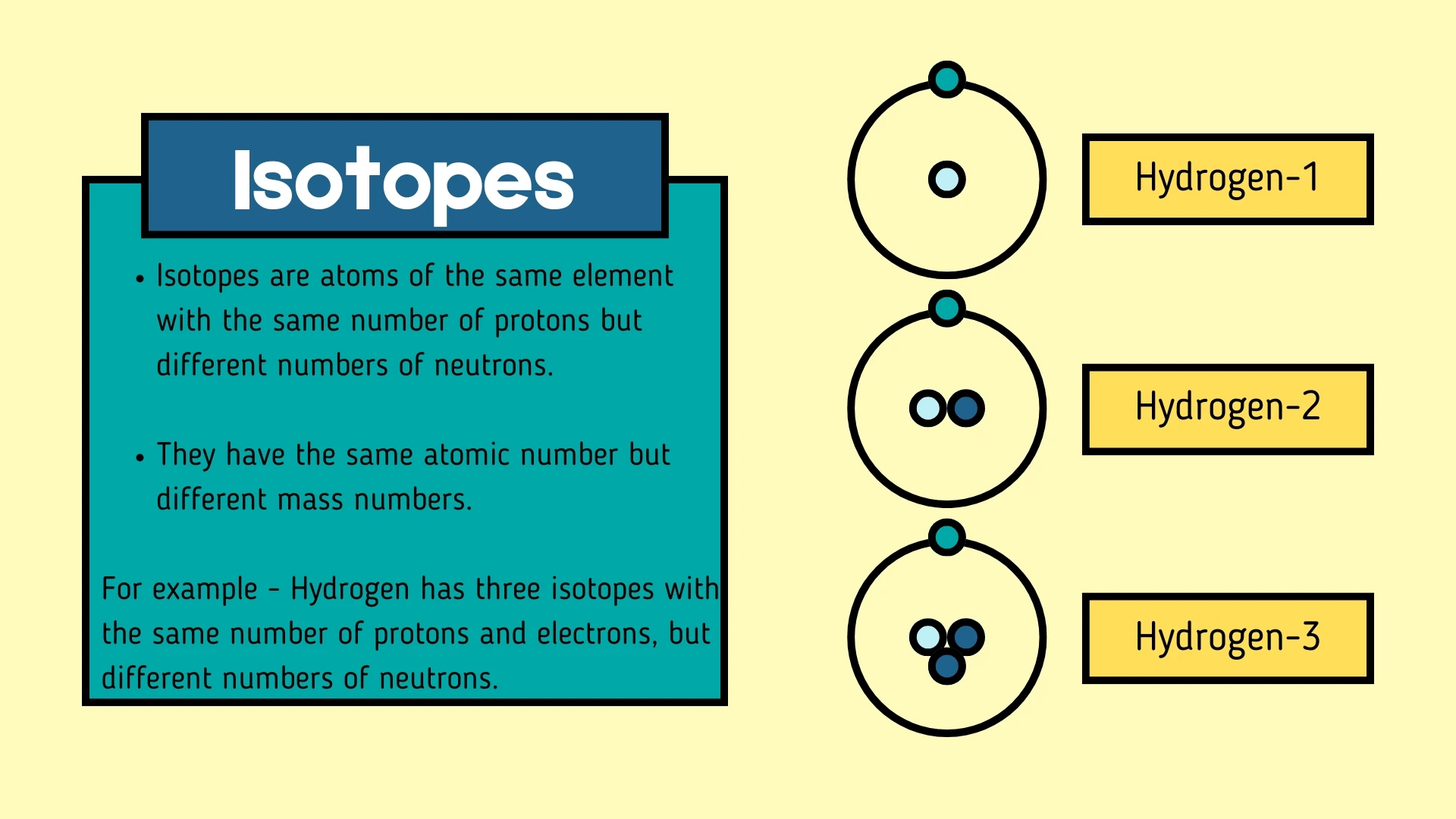
Isotope
Click to revealAtoms of the same element with the same number of protons but different numbers of neutrons. They have the same atomic number but different mass numbers.