📚 Lesson: History of Atomic Models
Introduction to Atomic Models – The Evolution of Our Understanding of the Atom
Atoms are the fundamental building blocks of matter. Every substance in the universe, whether it exists as a solid, liquid, gas or plasma, is made up of atoms. These particles are incredibly small and cannot be seen even with the most powerful optical microscopes. Our understanding of what atoms are and how they behave has developed over many centuries, shaped by the discoveries, experiments and ideas of scientists from different periods.
The concept of atomic models refers to the visual or conceptual ways used to explain the structure of atoms and the arrangement of their subatomic particles. Each model reflects the scientific thinking of its time and shows how knowledge evolved as new evidence became available. This study note explores the journey of atomic models, from the earliest ideas to the modern quantum mechanical model. It outlines how each model was proposed, its main features, its limitations and how it contributed to the growing scientific understanding of atomic structure.
This lesson is designed for students studying GCSE, IGCSE, CBSE, IB, and the AP Curriculum, specifically those in Grades 9 to 11. It includes informative notes, interactive activity sheets, flashcards, and engaging learning tasks to make this topic more enjoyable and easier to understand.
Why Atomic Models Matter
The idea of the atom began over 2,000 years ago in ancient Greece, when thinkers like Democritus suggested that all matter was made of tiny, indivisible particles called "atomos." This idea remained philosophical for centuries because there was no experimental proof.
As science developed, experiments in chemistry and physics showed that matter is made of elements and compounds, supporting the idea that atoms are real. Since atoms cannot be seen directly, scientists created models to explain their structure. Each model was based on new evidence that improved or replaced earlier ideas.
An atomic model is not just a picture; it is a scientific explanation of how atoms behave, how they join together and how they interact with energy and matter. Understanding these models helps students learn important chemistry topics such as bonding, periodic trends and chemical reactions.
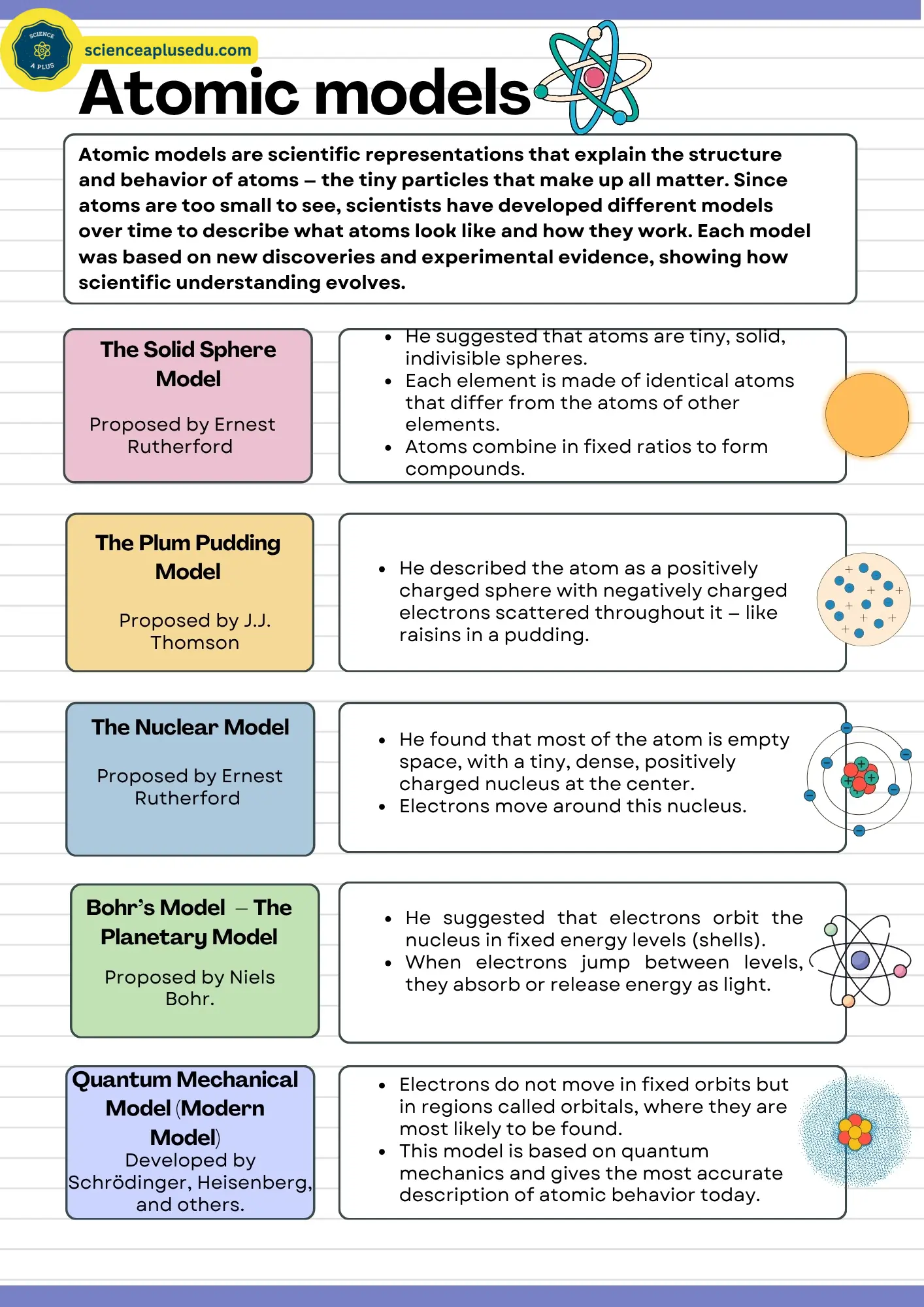
1. Dalton's Atomic Model (1803) – The Solid Sphere Model
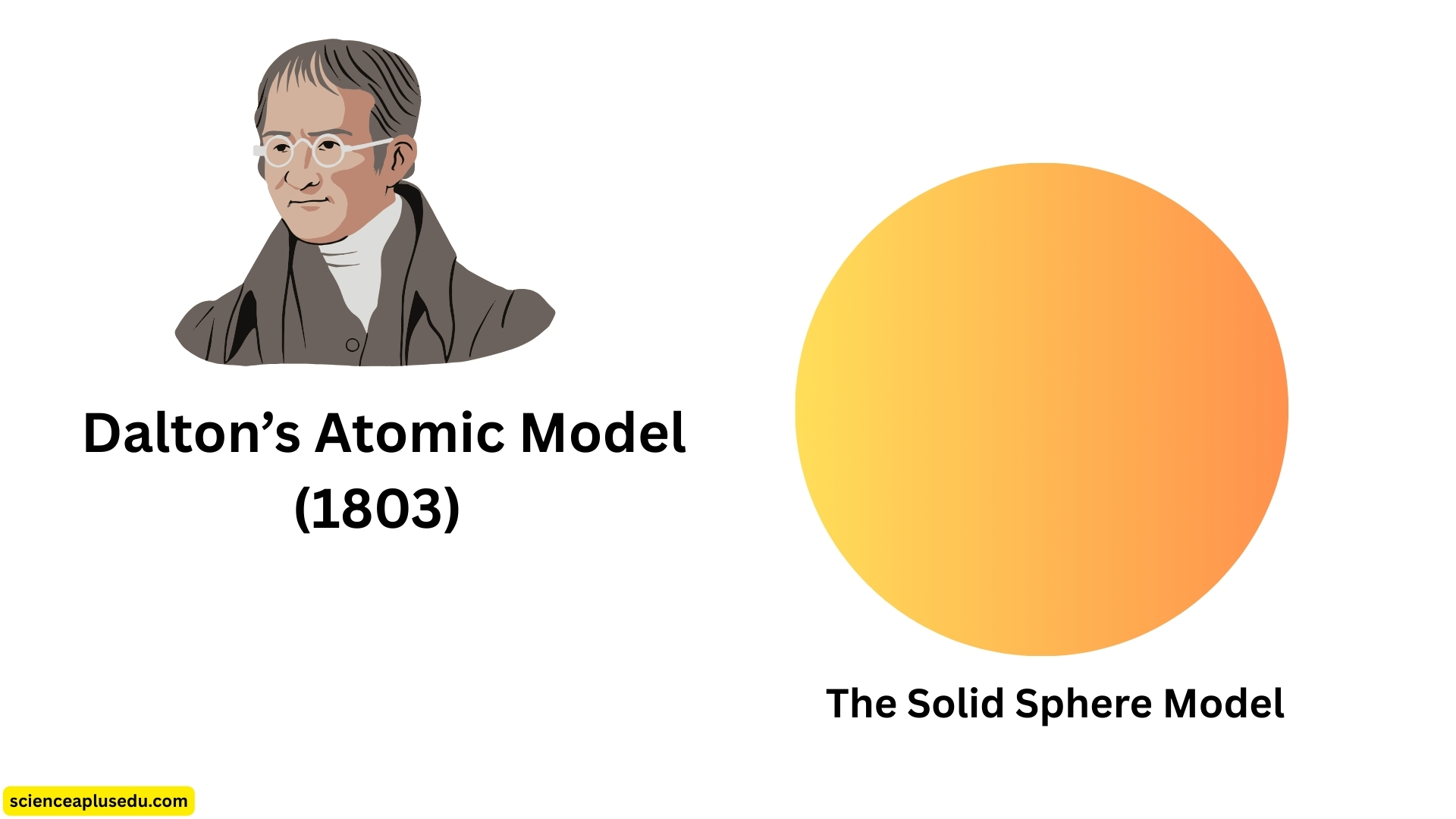
Dalton's Atomic Model
The first modern atomic theory
Background
In the early 19th century, chemical reactions were being studied extensively. Scientists noticed that elements always combined in fixed ratios by mass, a principle now known as the law of definite proportions. Based on these ideas, John Dalton, an English schoolteacher and chemist, proposed the first scientific theory of the atom in 1803. Dalton's Atomic Model (1803) suggested that all matter is made up of tiny, solid, indivisible, and indestructible particles called atoms.
Main Features
Dalton's atomic theory described atoms as:
- Tiny, indivisible particles that make up all matter
- Atoms of the same element are identical in mass and properties
- Atoms of different elements are different in mass and properties
- Atoms combine in simple whole-number ratios to form compounds
- Atoms cannot be created or destroyed during chemical reactions; they only rearrange
Strengths
Dalton's model explained the law of conservation of mass and the law of multiple proportions. It also provided a scientific basis for understanding chemical combinations.
Limitations
However, Dalton's model had weaknesses:
- It considered atoms to be indivisible, but later discoveries showed that atoms contain smaller particles — electrons, protons, and neutrons
- It did not explain how atoms combine or what happens inside them during reactions
Despite its limitations, Dalton's model laid the foundation for modern atomic theory.
2. Thomson's Atomic Model (1897) – The Plum Pudding Model
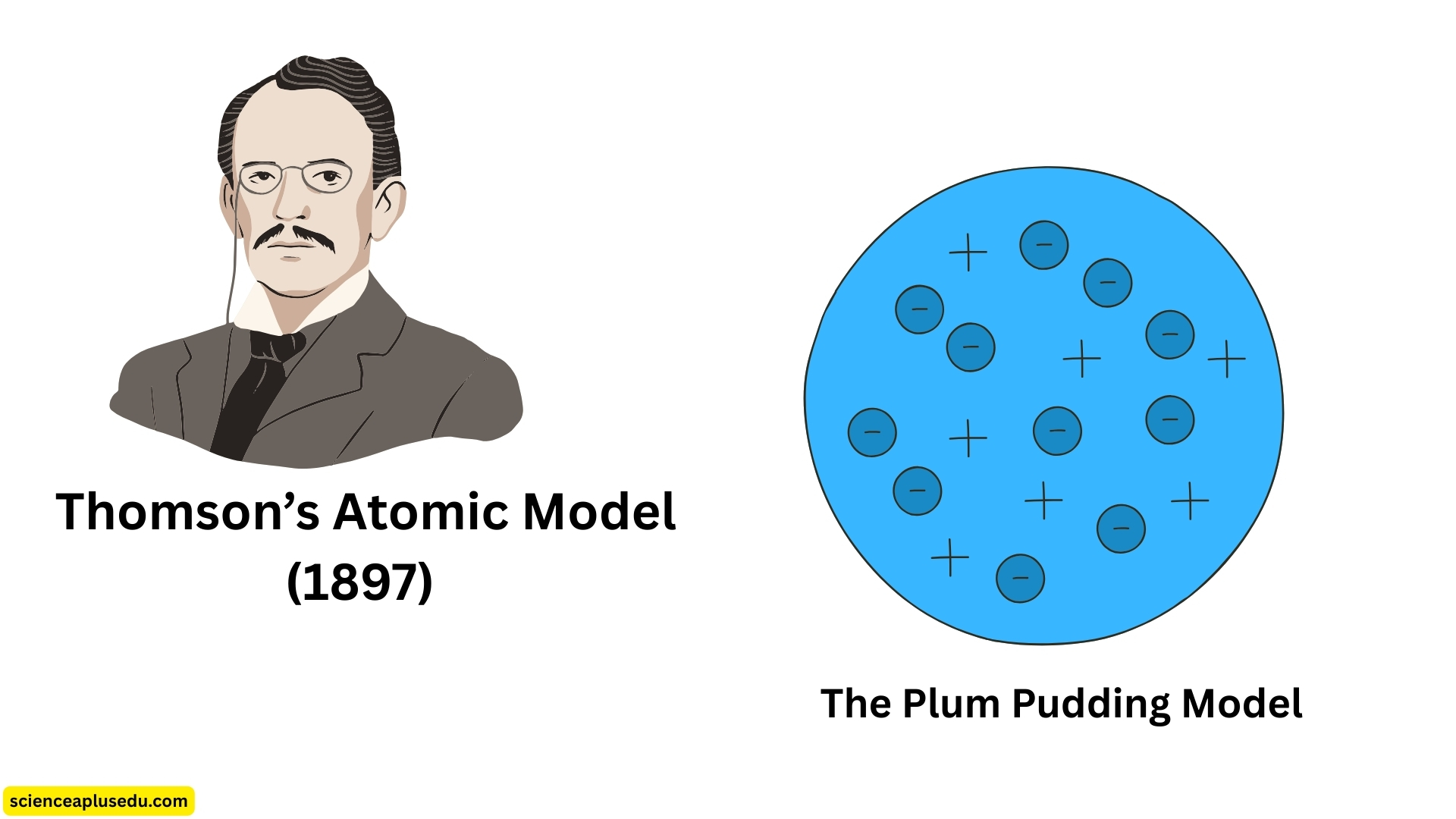
Thomson's Plum Pudding Model
First model to include electrons
Discovery of the Electron
In 1897, J.J. Thomson conducted experiments using cathode rays and discovered that atoms contained tiny, negatively charged particles — later named electrons. This discovery proved that atoms were not indivisible as Dalton had thought.
Description of the Model
Thomson proposed that:
- The atom is a positively charged sphere with negatively charged electrons embedded within it
- The positive and negative charges are evenly distributed, making the atom electrically neutral
- The atom is divisible, containing smaller particles
This idea became known as the "plum pudding model" because it resembled a pudding (the positive sphere) with plums (electrons) scattered throughout.
Limitations
While the model explained the overall neutrality of atoms, it had significant flaws:
- It did not describe how the positive charge was held together or how electrons were arranged
- It could not explain experimental results that came later, especially Rutherford's gold foil experiment
Thomson's discovery of the electron was revolutionary, but his atomic model was soon replaced by a more accurate one.
3. Rutherford's Atomic Model (1911) – The Nuclear Model
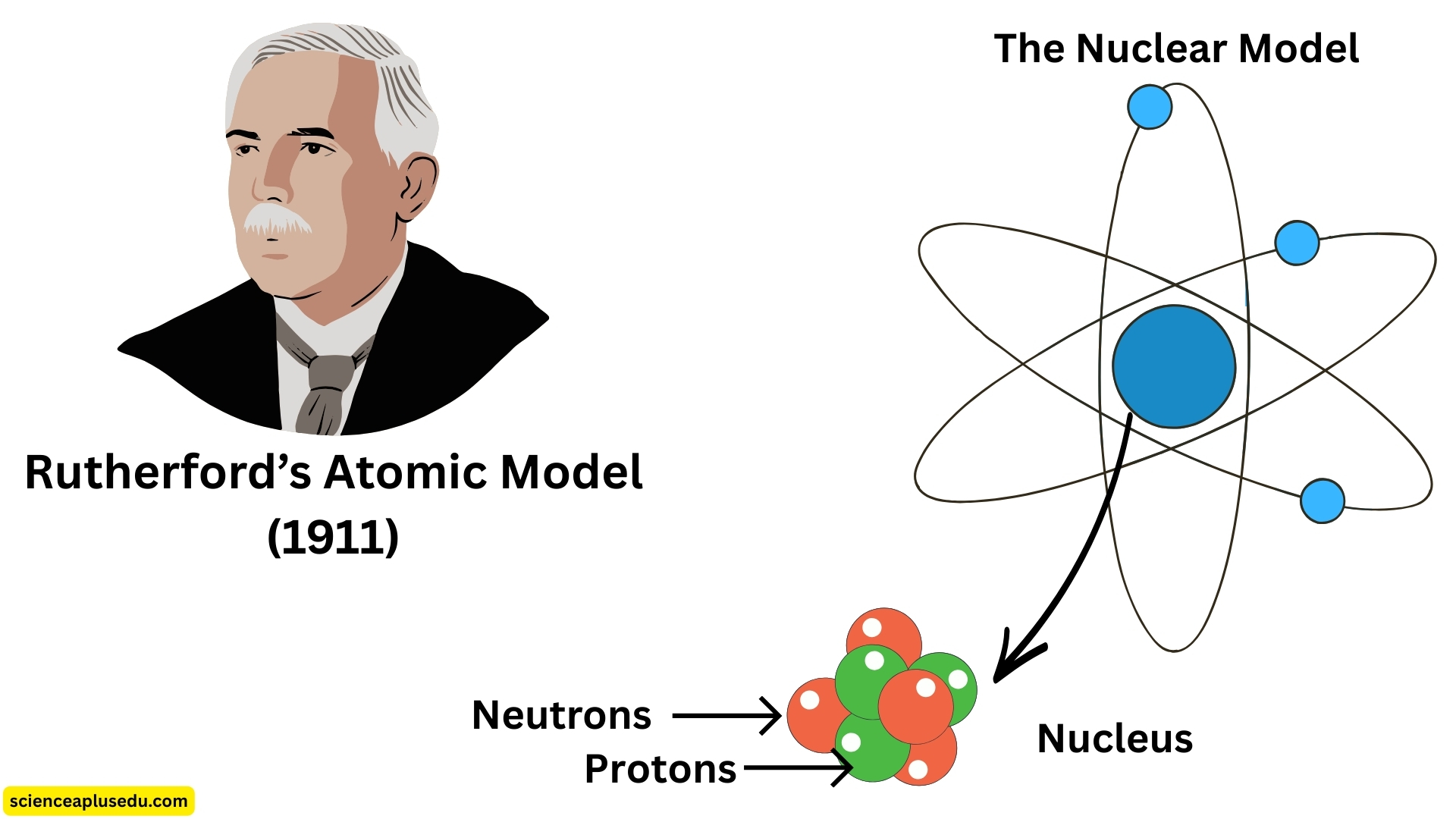
Rutherford's Nuclear Model
Discovered through the gold foil experiment
Gold Foil Experiment Animation
Watch how Rutherford discovered the nucleus
The Gold Foil Experiment
In 1911, Ernest Rutherford and his team (Hans Geiger and Ernest Marsden) conducted an experiment in which they fired alpha particles (positively charged helium nuclei) at a thin sheet of gold foil. They observed that most particles passed straight through, but a few were deflected or even bounced back.
Observations and Conclusions
From these results, Rutherford concluded:
- Most of the atom is empty space, allowing alpha particles to pass through
- A small, dense, positively charged nucleus exists at the center, causing deflections
- Electrons move around this nucleus like planets orbiting the sun
- The nucleus contains protons and neutrons
This was known as the nuclear model of the atom.
Strengths
- It explained the existence of a nucleus and provided evidence for atomic structure
- It overturned the idea of the atom as a uniform sphere
Limitations
- Rutherford's model could not explain why electrons do not spiral into the nucleus due to electrostatic attraction
- It could not describe how atoms emit or absorb light
Rutherford's model was an essential step forward, introducing the concept of the nucleus, which remains central to atomic theory today.
4. Bohr's Atomic Model (1913) – The Planetary Model
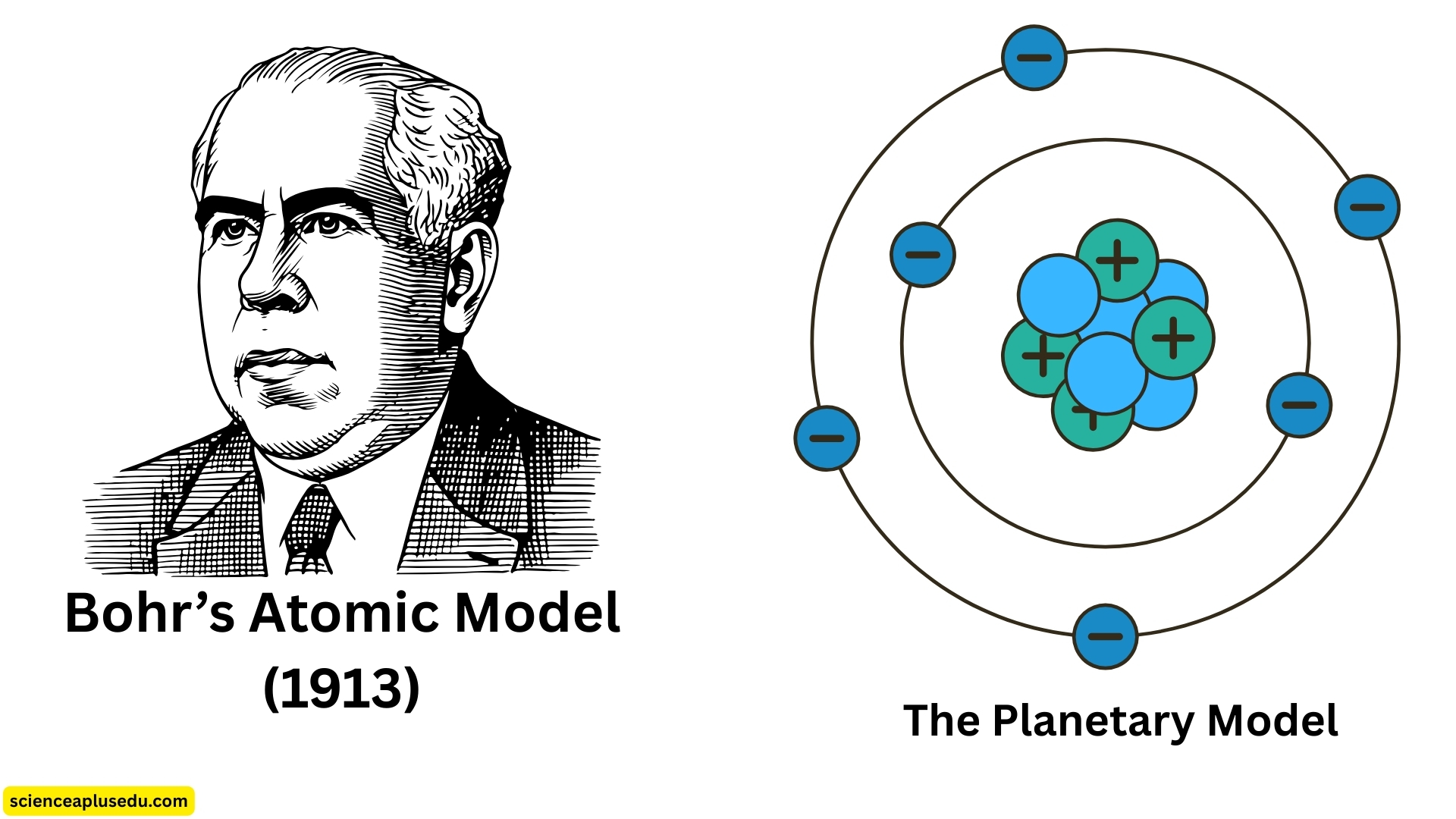
Bohr's Planetary Model
Electrons in fixed energy levels
Electron Energy Levels Animation
See how electrons jump between shells
Background
Building on Rutherford's model, Niels Bohr proposed a new model in 1913. He studied the hydrogen emission spectrum — the light emitted when excited hydrogen atoms returned to lower energy levels. Bohr realized that the spectral lines corresponded to specific energy levels of electrons.
Key Features
- Electrons orbit the nucleus in fixed energy levels (shells)
- Each orbit has a specific energy, and electrons can move between levels by absorbing or emitting energy in the form of photons
- The energy of the photon equals the difference between the two energy levels
- Each shell can hold a specific maximum number of electrons:
- 1st shell → up to 2 electrons
- 2nd shell → up to 8 electrons
- 3rd shell → up to 18 electrons
Example: Hydrogen Spectrum
When a hydrogen atom's electron jumps from the second to the first energy level, it emits light of a specific wavelength (in the ultraviolet region). This explained why only certain colors appeared in hydrogen's emission spectrum.
Strengths
- Explained atomic spectra for hydrogen accurately
- Introduced the concept of quantized energy levels, which remains fundamental in quantum theory
Limitations
- Could not explain spectra for multi-electron atoms
- Failed to describe the fine details observed in spectral lines
- The model assumed circular orbits, which were later proven incorrect
Despite its limitations, Bohr's model successfully introduced quantum concepts into atomic theory.
5. The Quantum Mechanical Model (1926 – Present)
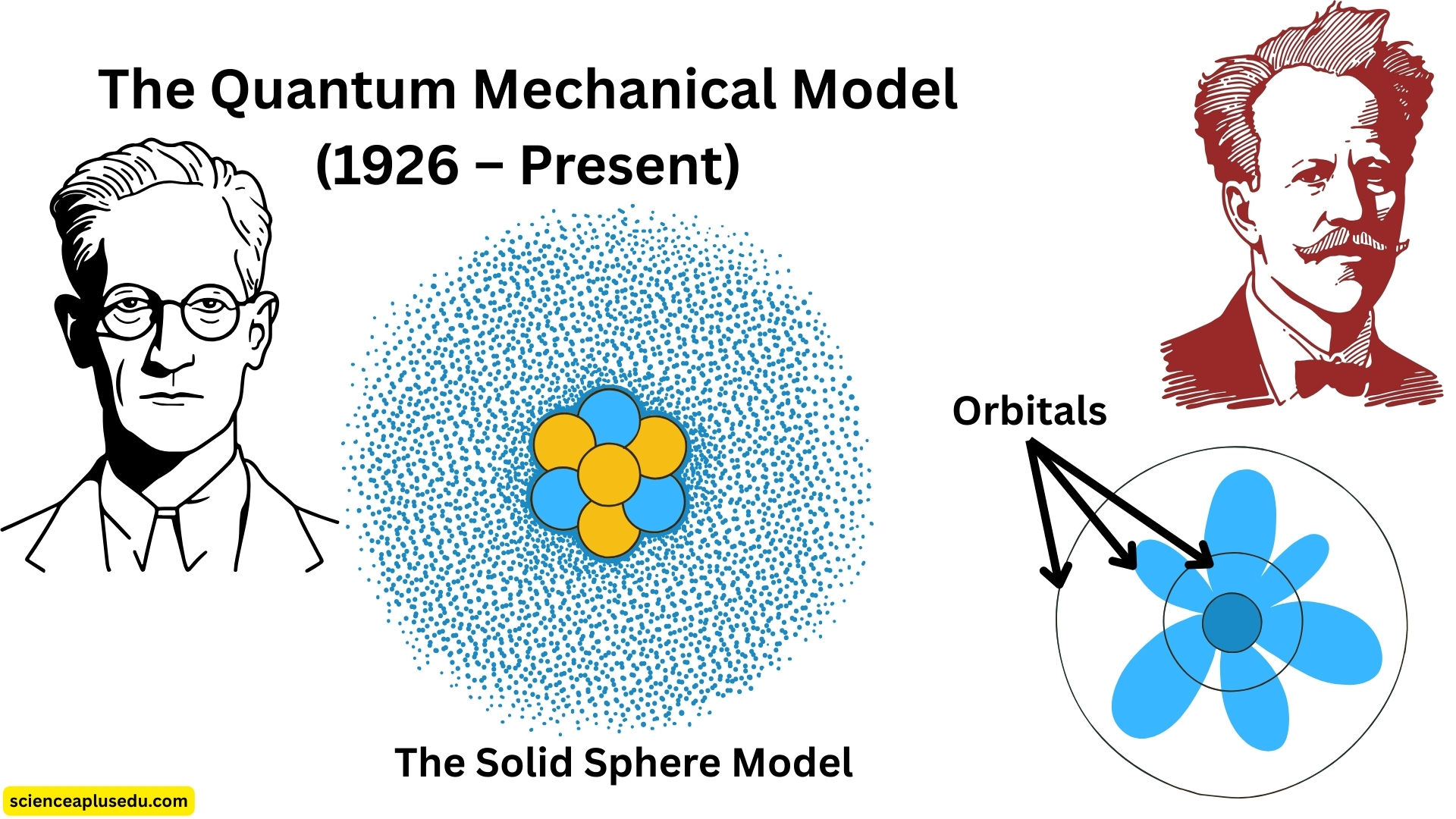
Quantum Mechanical Model
The modern atomic model
Development
With advances in quantum physics, scientists such as Erwin Schrödinger, Werner Heisenberg, and Louis de Broglie developed a new model that combined particle and wave theories of matter.
Main Concepts
- Electrons behave both as particles and waves (wave-particle duality)
- The exact position of an electron cannot be determined — only the probability of finding it in a certain region
- These regions are called orbitals, which describe where electrons are most likely to be found
- Schrödinger's wave equation mathematically defines the shapes and energies of these orbitals
Electron Cloud Concept
Instead of fixed paths, electrons form a cloud around the nucleus. Denser regions in the cloud represent a higher probability of finding an electron. We can only calculate the probability of finding an electron in a certain location.
Strengths
- Explains behavior of all atoms, including complex ones
- Accurately predicts chemical bonding and spectral behavior
- Forms the basis of modern chemistry and quantum mechanics
Limitations
- Difficult to visualize; relies on advanced mathematics
- Does not give definite positions for electrons
Why it's important
The quantum mechanical model remains the most accurate and widely accepted model of the atom today. This model forms the foundation of modern chemistry and physics, explaining chemical bonding, molecular structure, and the behavior of elements.
Summary Comparison
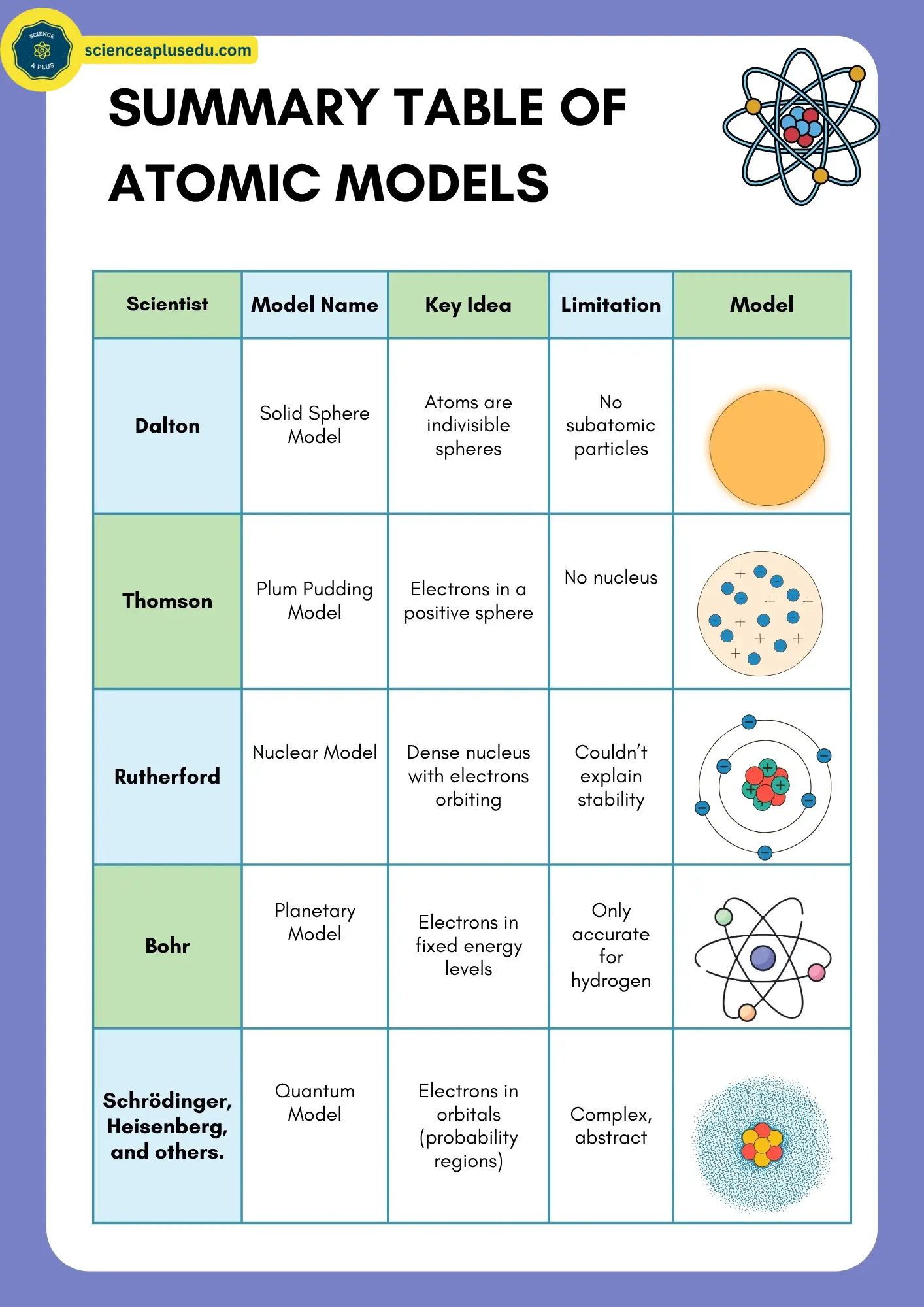
Atomic Models Summary Table
Complete comparison of all models
Revision Notes
Quick reference guide
This table highlights how atomic theory progressed from simple to complex, with each model addressing the shortcomings of the previous one.
Importance of Atomic Models in Modern Science
Understanding atomic models is vital for many areas of science:
- Chemistry: Explains bonding, molecular structure, and reactions
- Physics: Provides the basis for quantum mechanics and nuclear physics
- Biology: Helps explain biochemical interactions at the molecular level
- Engineering and Technology: Essential for developing materials, semiconductors, and nanotechnology
Atomic theory continues to influence research in nuclear energy, medical imaging, and quantum computing, demonstrating its enduring importance.
Conclusion
The journey of atomic models, from Dalton's solid spheres to Schrödinger's quantum model, shows how human understanding has grown through observation, experimentation and reasoning. Each model added new insight, helping scientists explain the invisible world of atoms more accurately.
Modern atomic theory is the result of centuries of scientific progress. It reminds us that science continually develops as new evidence appears. The story of atomic models is not only about atoms but also about human curiosity and the search for knowledge.
🎴 Flashcards: Quick Revision
Who proposed the Solid Sphere Model?
Click to flipJohn Dalton proposed the Solid Sphere Model in 1803, suggesting atoms are indivisible solid spheres.
What did J.J. Thomson discover?
Click to flipThomson discovered the electron in 1897 and proposed the Plum Pudding Model where electrons are embedded in a positive sphere.
What experiment led to Rutherford's model?
Click to flipThe Gold Foil Experiment in 1911, where alpha particles were fired at gold foil, revealing the nucleus.
What are energy levels in Bohr's model?
Click to flipFixed circular paths (shells) where electrons orbit the nucleus at specific distances, with each level holding a maximum number of electrons.
What is the main limitation of Dalton's model?
Click to flipIt did not account for subatomic particles like protons, neutrons, and electrons.
What are orbitals?
Click to flipRegions in the Quantum Mechanical Model where electrons are most likely to be found, based on probability rather than fixed paths.
Why is most of the atom empty space?
Click to flipRutherford's experiment showed that most alpha particles passed through gold foil, proving atoms are mostly empty with a tiny dense nucleus.
What happens when electrons jump energy levels?
Click to flipElectrons absorb energy when moving to higher levels and release energy (as light) when dropping to lower levels.
Who developed the current atomic model?
Click to flipSchrödinger, Heisenberg, de Broglie, and others developed the Quantum Mechanical Model starting in 1926.
What is wave-particle duality?
Click to flipThe concept that electrons behave as both waves and particles, a key principle of quantum mechanics.
How many electrons can the 1st shell hold?
Click to flipThe first shell (K shell) can hold up to 2 electrons maximum.
What charge does the nucleus have?
Click to flipThe nucleus has a positive charge due to protons, discovered by Rutherford in 1911.
📝 Quiz: Test Your Knowledge
Your Score: 0/10
📄 Worksheets: Practice & Review
Activity Sheets Set 1: Filling Table
Match atomic model descriptions with scientists and years. Includes answer sheet.
Download Collection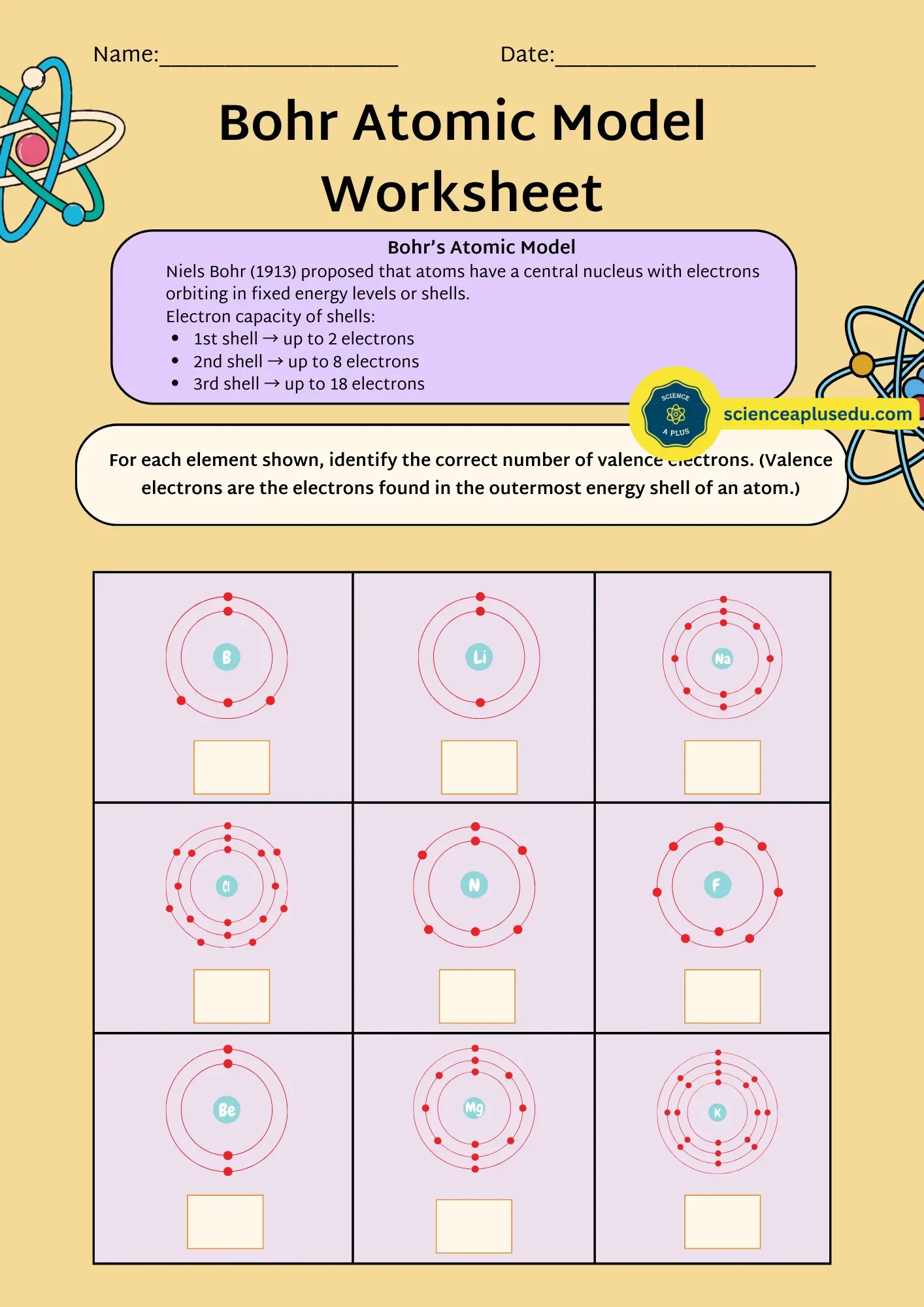
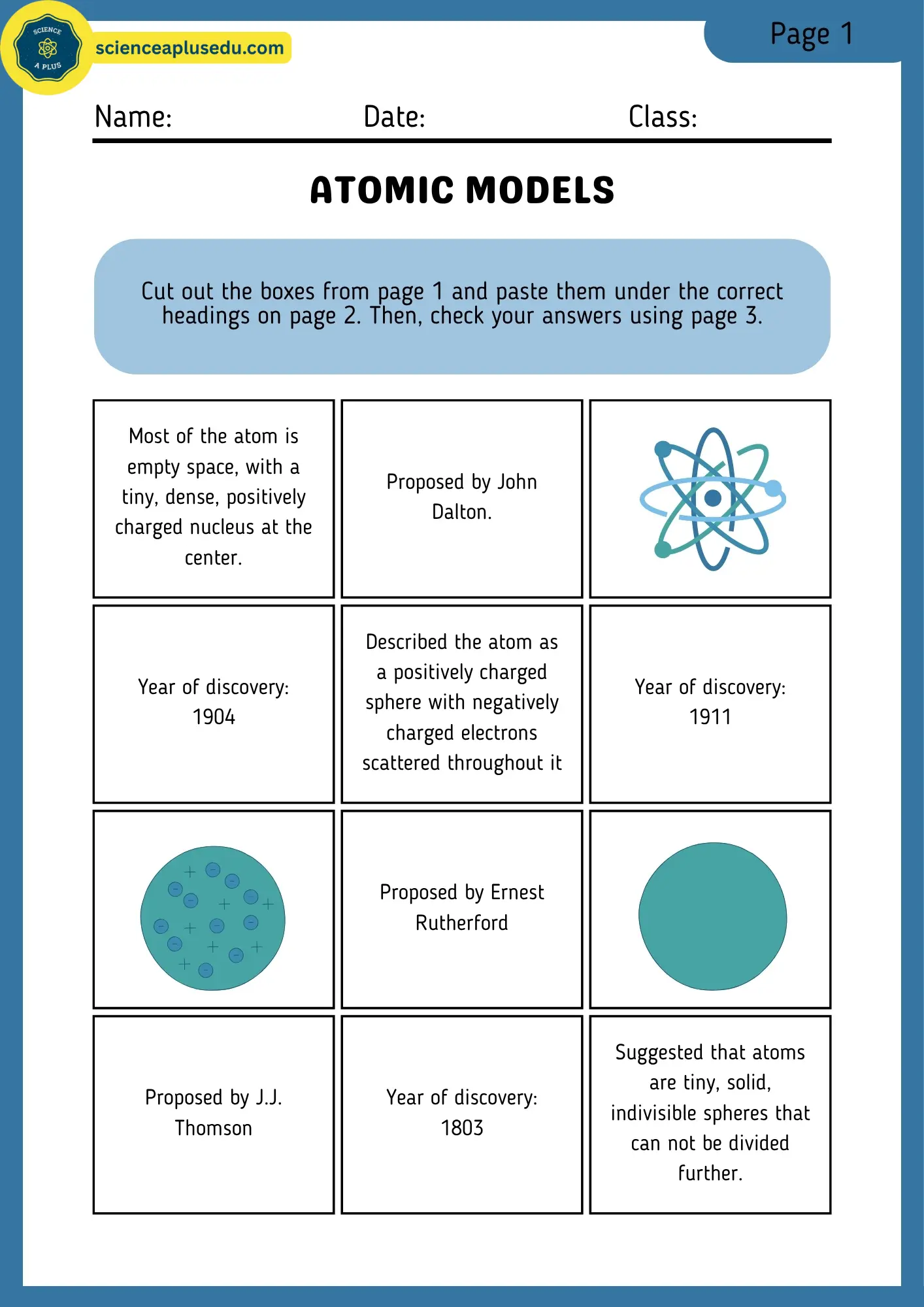
Activity Sheets Set 2: Filling Boxes
Cut and paste activity to sort atomic models. Complete with answer key.
Download Collection