📖 Lesson: Acids, Bases & Alkalis
Explore the chemistry of acids, bases, alkalis, and neutralisation reactions
🎮 Interactive Activities
Test your knowledge with these fun activities
🔗 Match the Terms
Click a term, then click its matching definition!
✏️ Fill in the Blanks
Click on a word from the word bank, then click on the blank to fill it
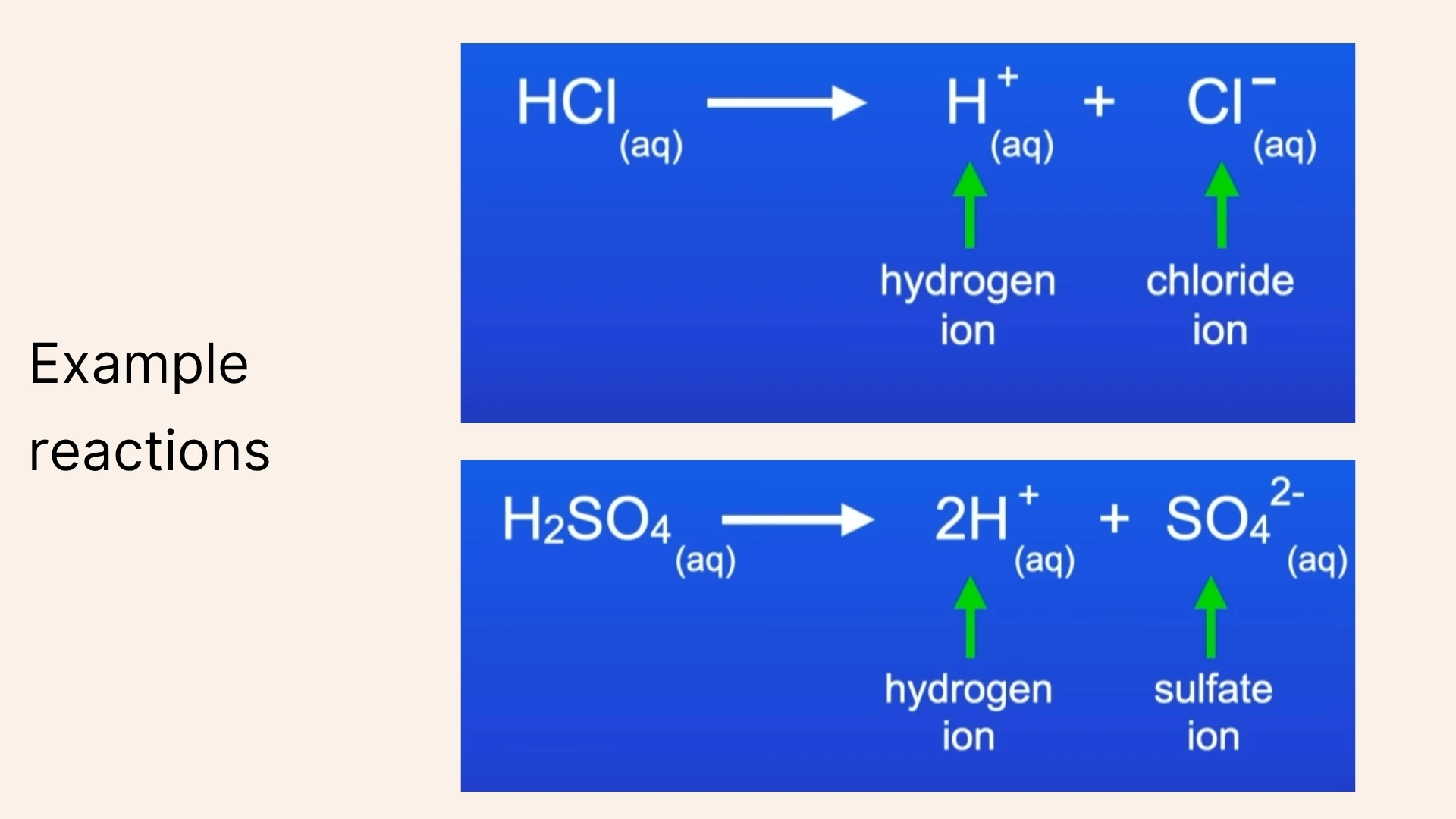
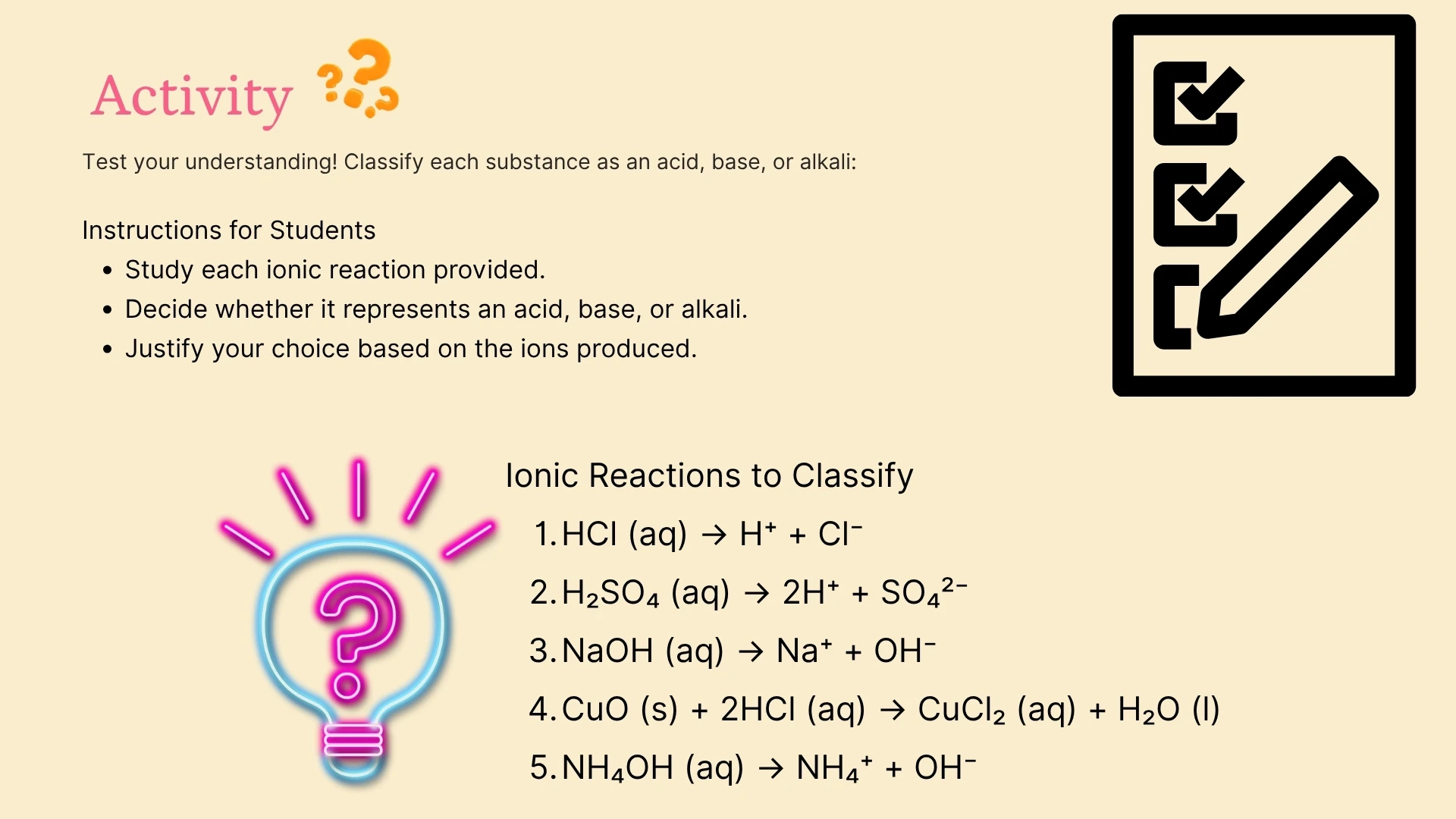
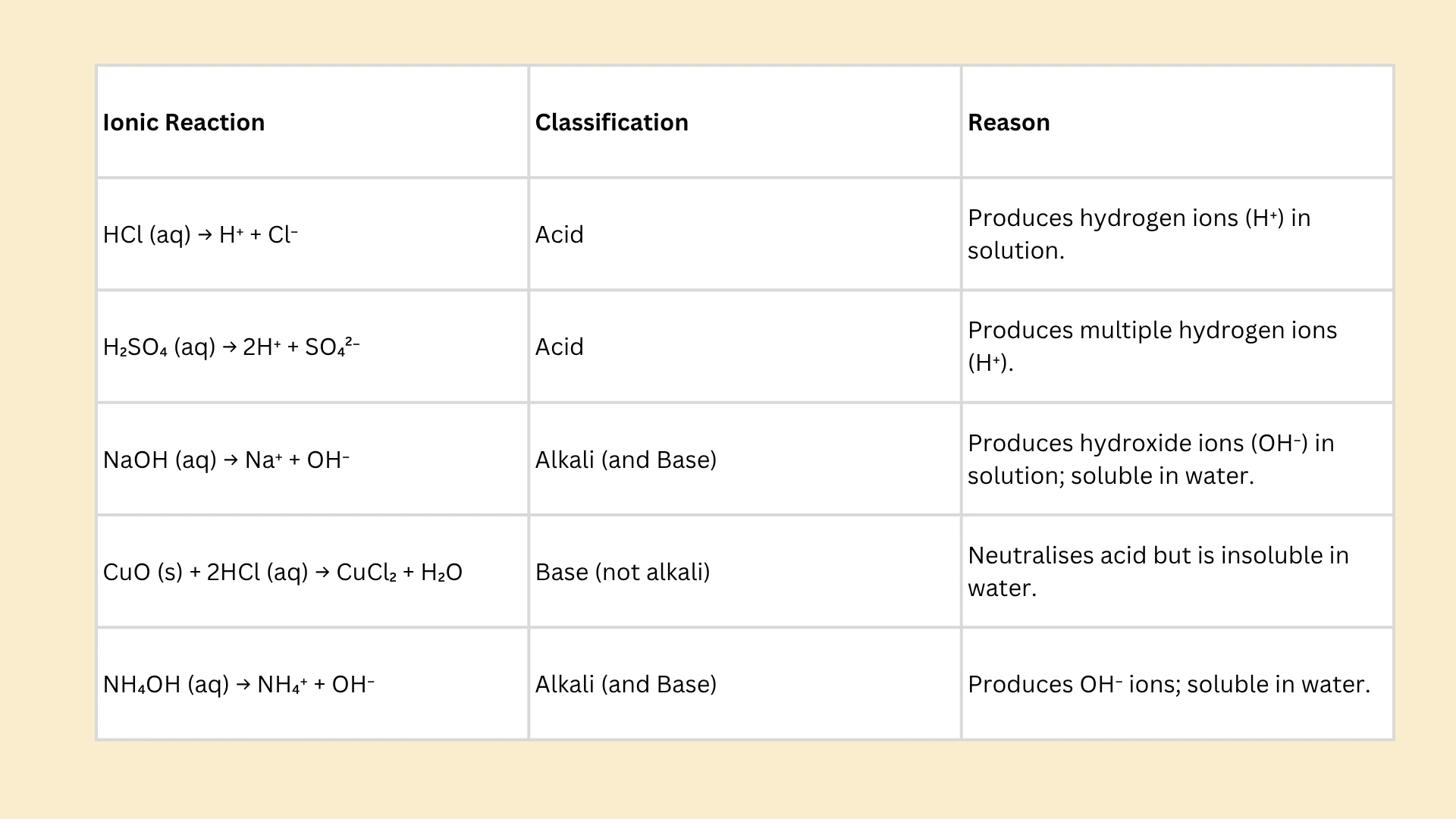
1. Acids produce ions (H⁺) when dissolved in water.
2. Alkalis produce ions (OH⁻) in solution.
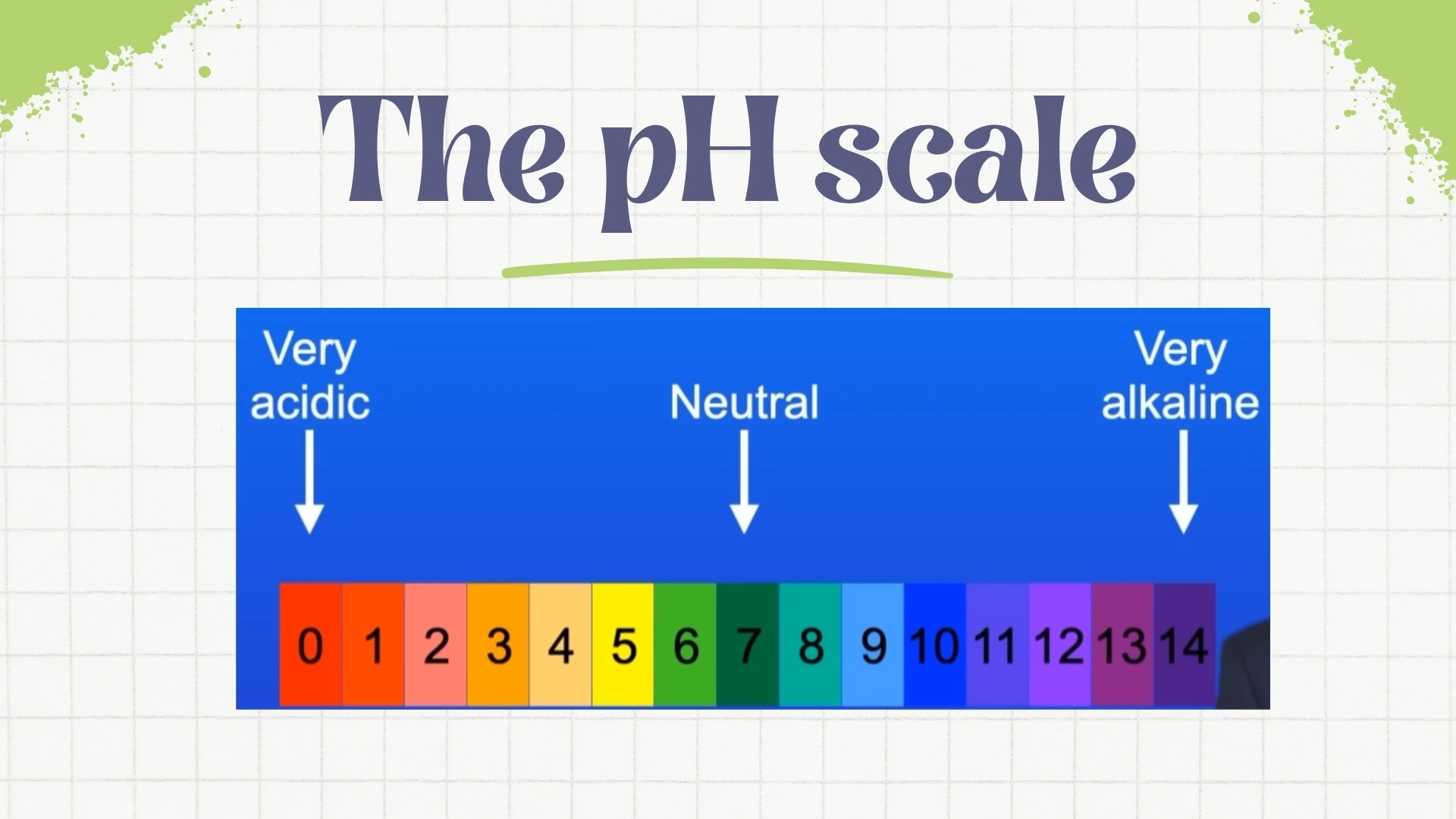
3. A neutral substance has a pH of .
4. When an acid reacts with a base, the products are and .
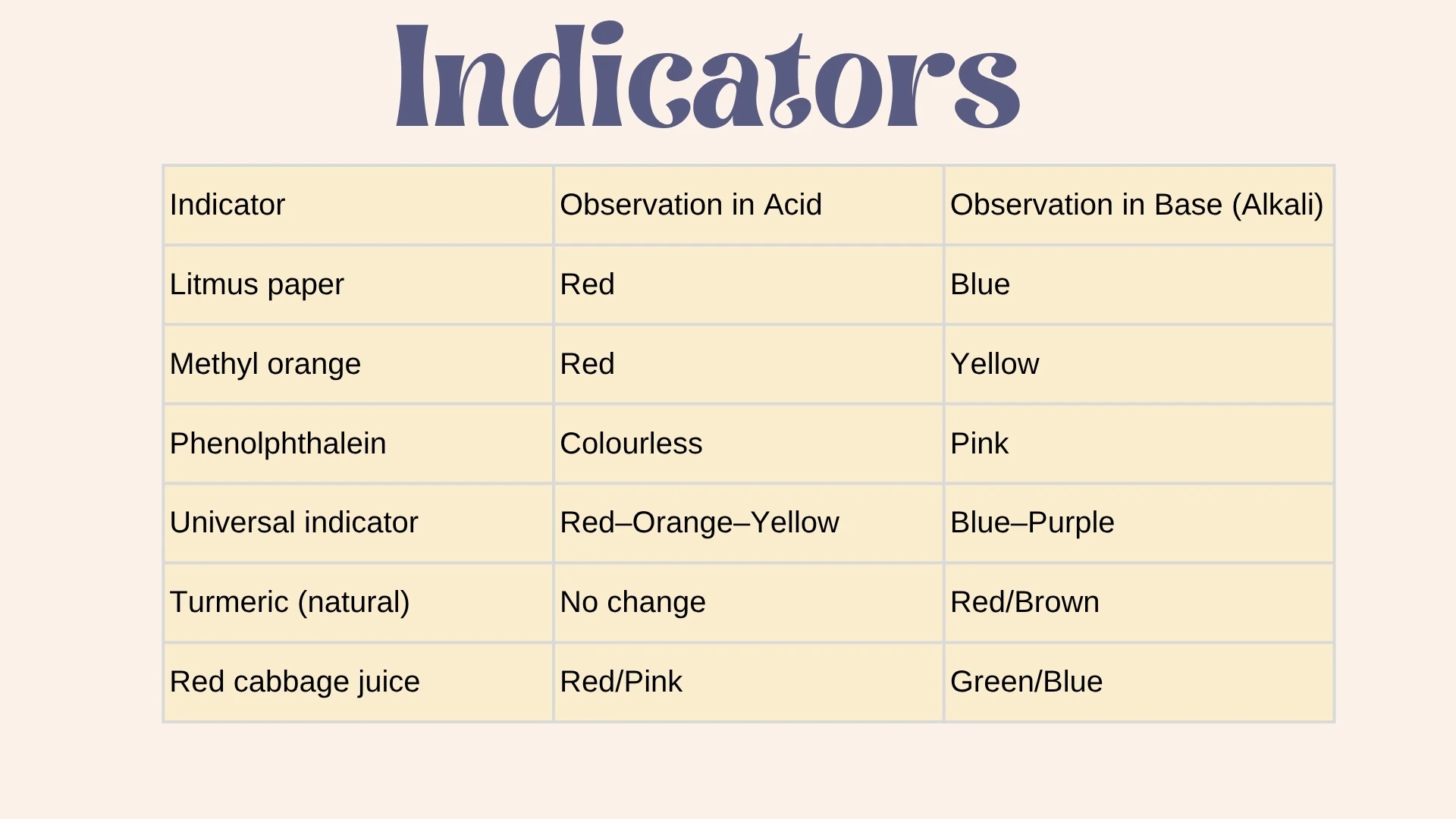
5. Acids turn blue litmus paper .
6. Alkalis turn red litmus paper .
7. The reaction between an acid and a base is called .
You got 0/8 correct!
📚 Flashcards
Click on each card to reveal the definition
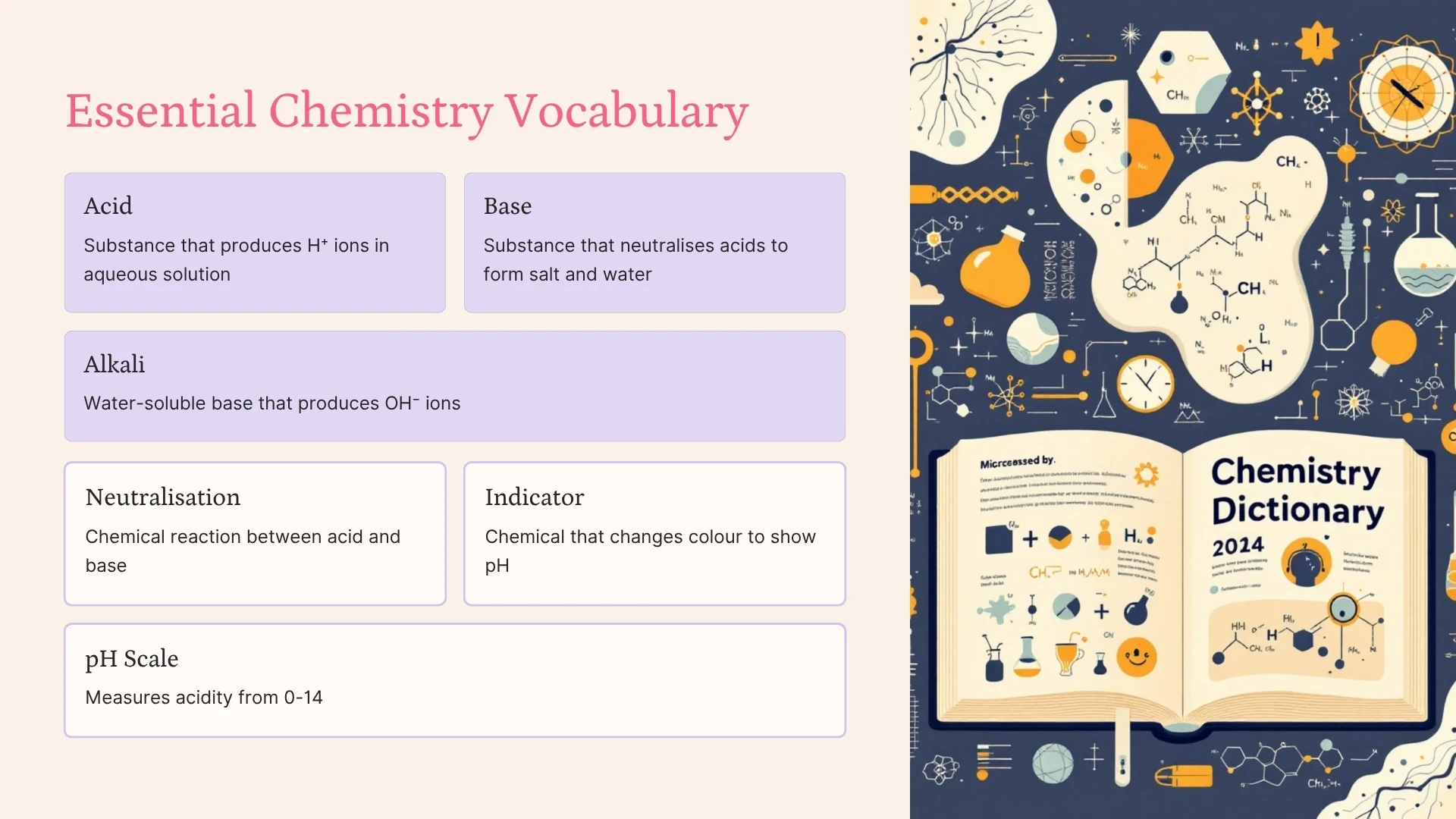
Acid
Click to revealA substance that produces hydrogen ions (H⁺) when dissolved in aqueous solution. Acids have a pH less than 7 and turn blue litmus paper red.
Base
Click to revealA substance that neutralises acids to form salt and water. Bases are the chemical opposite of acids. Examples include metal oxides and metal hydroxides.
Alkali
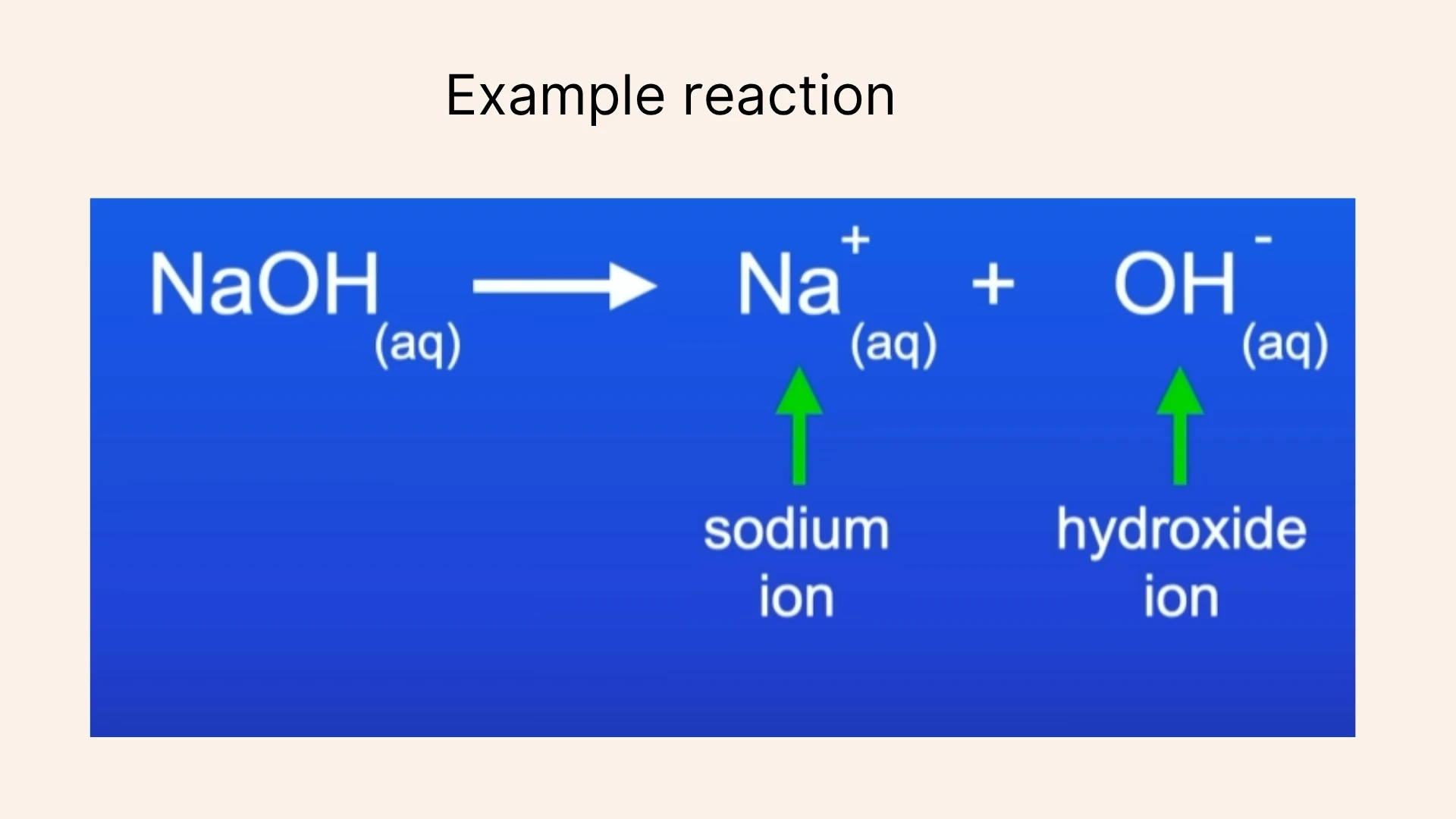
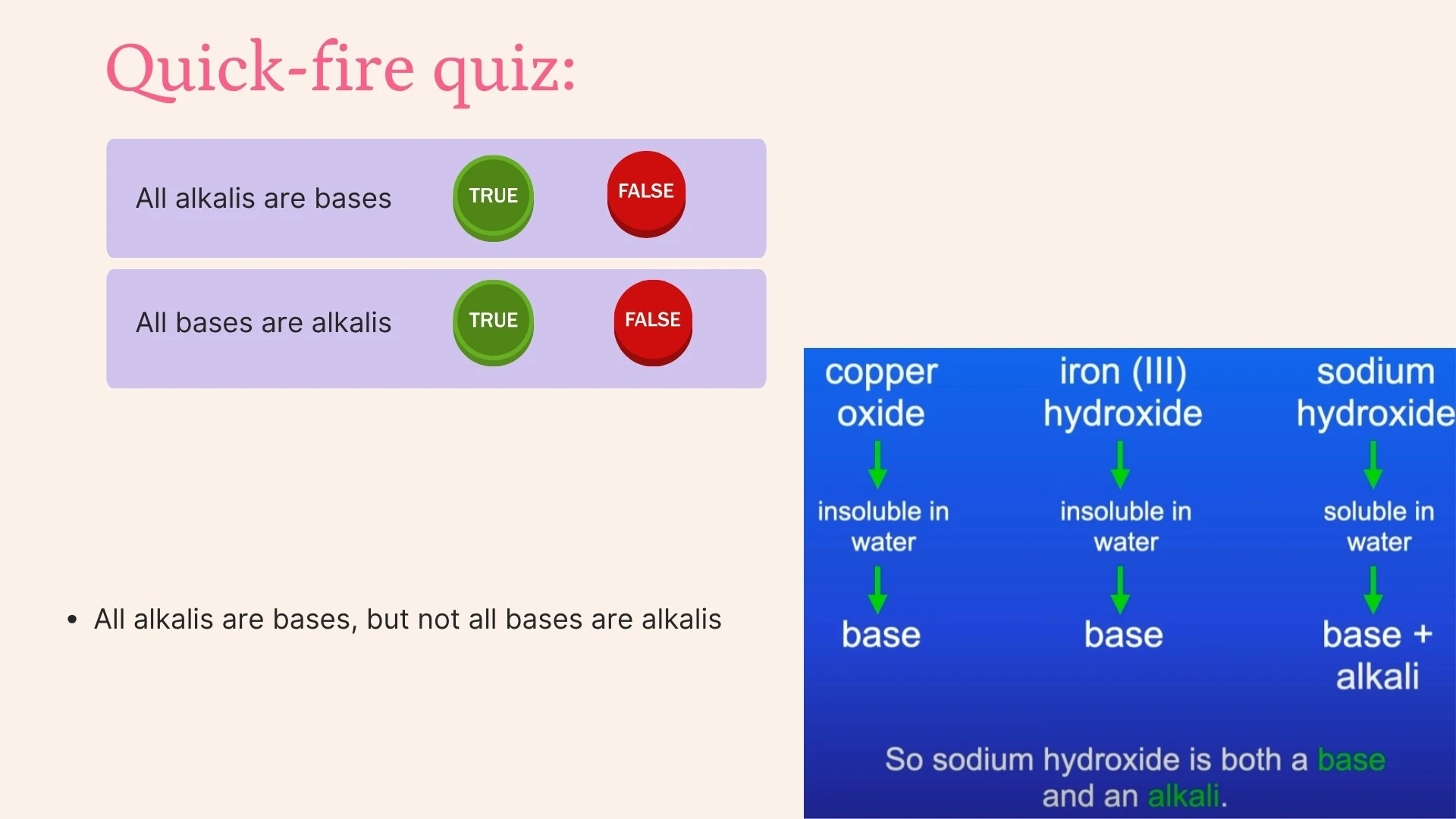
Click to revealA water-soluble base that produces hydroxide ions (OH⁻) in solution. All alkalis are bases, but not all bases are alkalis. pH is greater than 7.
pH Scale
Click to revealA scale from 0-14 that measures acidity or alkalinity. pH 0-6 is acidic, pH 7 is neutral, and pH 8-14 is alkaline. The lower the pH, the stronger the acid.
Indicator
Click to revealA chemical that changes colour to show pH. Examples: litmus paper (red/blue), universal indicator (rainbow colours), phenolphthalein (colourless/pink).
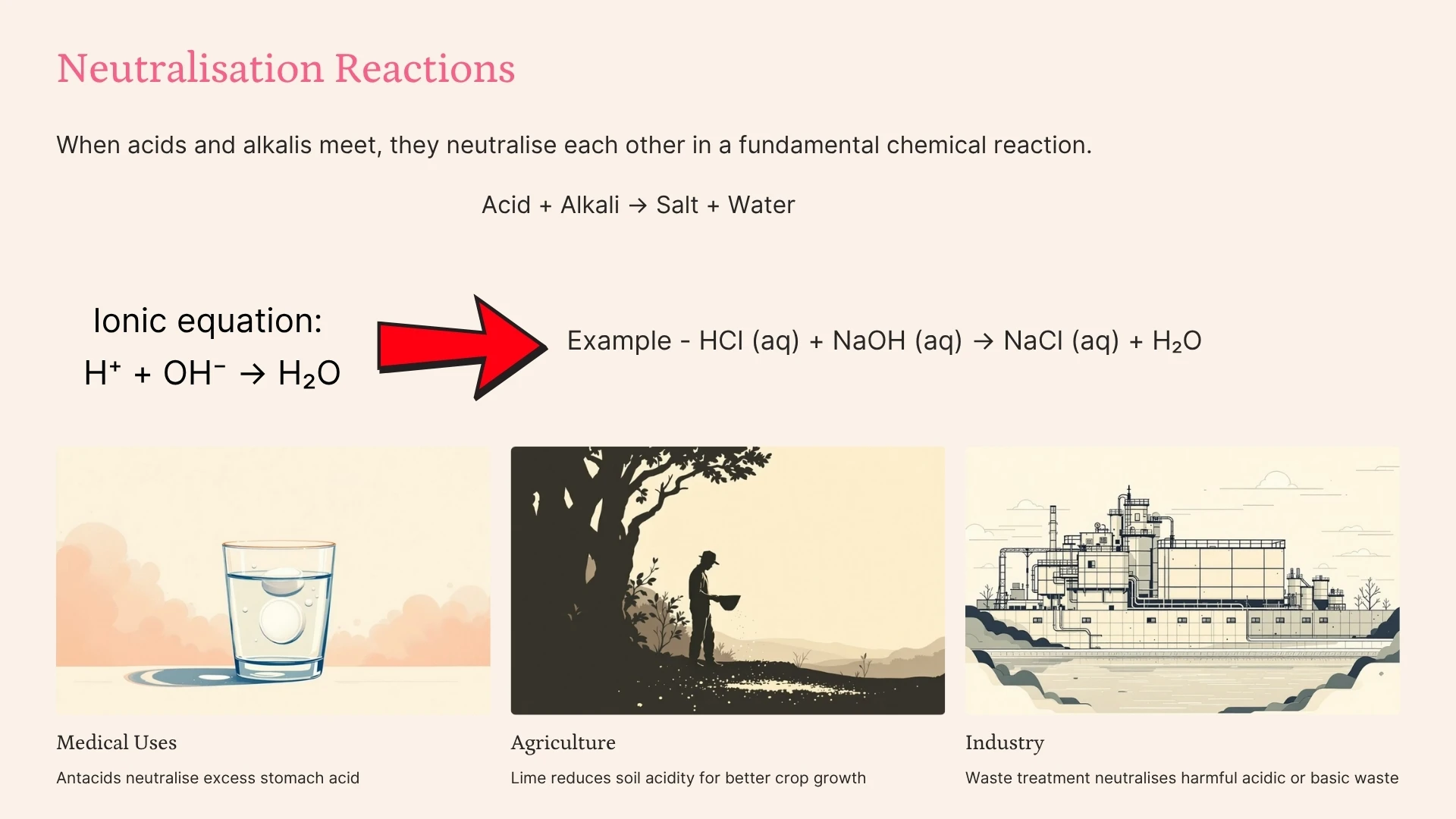
Neutralisation
Click to revealA chemical reaction between an acid and a base. The products are salt and water. Ionic equation: H⁺ + OH⁻ → H₂O
Hydrogen Ion (H⁺)
Click to revealA positively charged ion produced when acids dissolve in water. The presence of H⁺ ions gives acids their characteristic properties including sour taste.
Hydroxide Ion (OH⁻)
Click to revealA negatively charged ion produced when alkalis dissolve in water. The presence of OH⁻ ions gives alkalis their characteristic slippery feel.
Strong vs Weak Acids
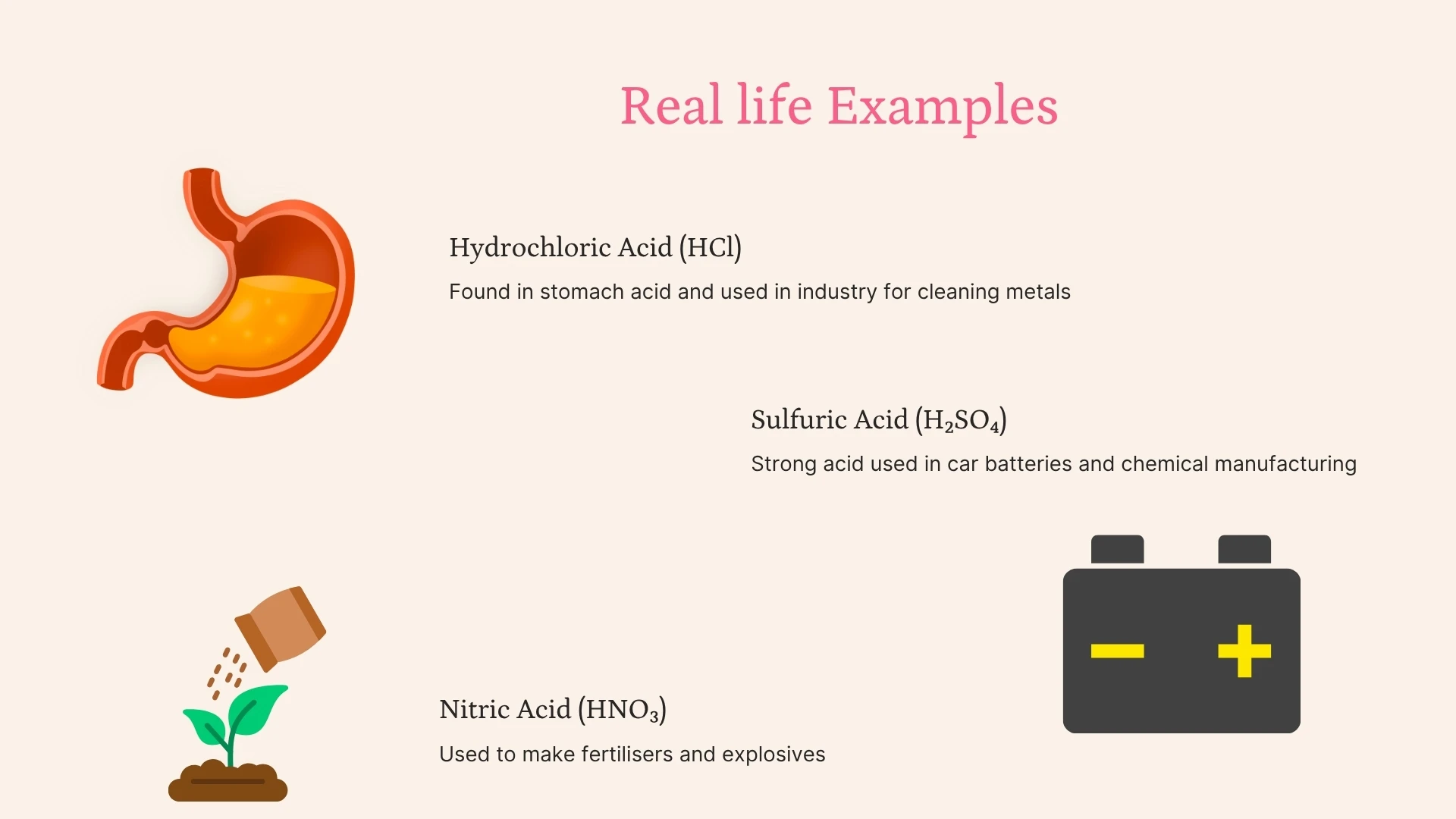
Click to revealStrong acids (HCl, H₂SO₄, HNO₃) fully dissociate in water. Weak acids (citric, ethanoic) only partially dissociate. Strength ≠ concentration!
🖱️ Interactive Simulations
Explore acids and bases through hands-on virtual experiments
pH Scale Simulation
Test the pH of different household substances. Add acids and bases to see how pH changes. Discover what makes something acidic or alkaline!
Launch SimulationAcid-Base Solutions Lab
Investigate how acids and bases interact with water. Explore the relationship between concentration, strength, and pH in this virtual laboratory.
Launch Simulation