📖 Introduction
Explore the chemical compounds that make up all living organisms
Living organisms are made of a wide range of chemical compounds formed by the bonding of naturally occurring elements in many different ways. Although there are ninety-two elements in nature, only about twenty-five are found in the human body, each located in different parts and present in different forms.
Among these, carbon, hydrogen, oxygen and nitrogen are the most abundant, while elements such as sulphur, phosphorous, sodium, potassium, calcium, magnesium, iron and chlorine also play vital roles in keeping organisms alive. In the human body, oxygen is the highest in percentage by mass, followed by carbon, hydrogen and nitrogen.
The chemical compounds that make up living matter fall into two main groups: organic compounds (containing carbon) and inorganic compounds (not containing carbon). The organic compounds that form the structure and functioning of living organisms are called biomolecules, which include carbohydrates, proteins, lipids and nucleic acids.
🍞 1. Carbohydrates
The primary source of energy for living organisms
What are Carbohydrates?
Carbohydrates are organic molecules composed of carbon, hydrogen, and oxygen, typically in a 1:2:1 ratio. They provide energy and structural support for living organisms.
The general formula for carbohydrates is: Cx(H2O)y
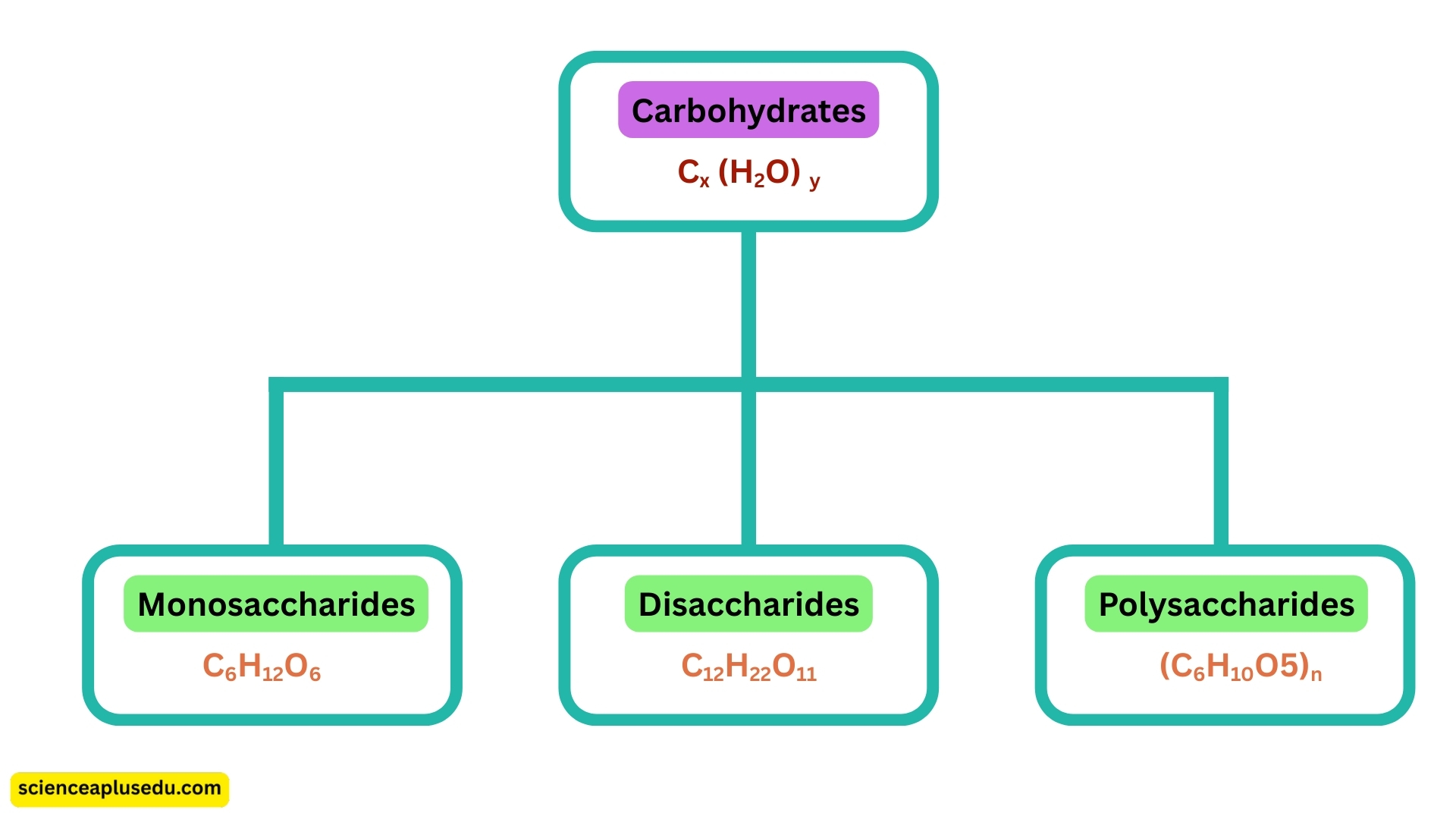
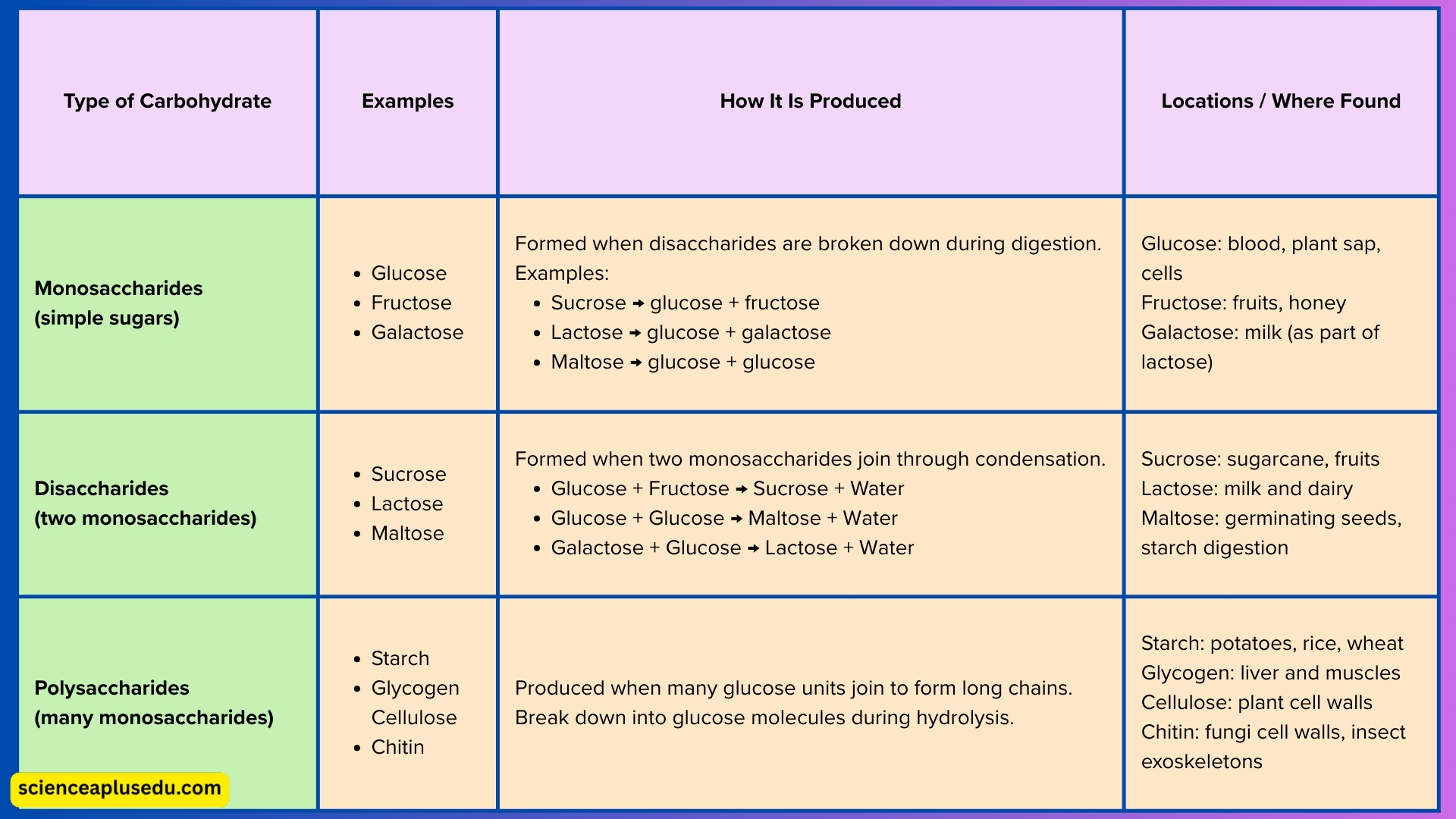
Types of Carbohydrates
1. Monosaccharides – Simple Sugars
- Smallest, basic units of carbohydrates with formula C6H12O6
- Easily absorbed and used quickly for energy
- Examples: Glucose (main cellular fuel), Fructose (fruit sugar), Galactose (part of lactose)
2. Disaccharides – Two Monosaccharides Joined
- Formed by combining two simple sugars through condensation with formula C12H22O11
- Must be broken into monosaccharides for absorption
- Examples: Sucrose (glucose + fructose), Lactose (glucose + galactose), Maltose (glucose + glucose)
3. Polysaccharides – Long Chains of Monosaccharides
- Large, complex carbohydrates made of many glucose units with formula (C6H10O5)n
- Provide storage or structural support depending on type
- Examples:
- Starch – plant storage carbohydrate
- Glycogen – animal storage carbohydrate stored in liver and muscles
- Cellulose – structural carbohydrate in plant cell walls
- Chitin – structural carbohydrate in fungi and arthropod exoskeletons
Functions of Carbohydrates
1. Energy Source
- Glucose is broken down during cellular respiration to release ATP
- Provides quick and efficient energy, especially for the brain and muscles
2. Energy Storage
- Plants store excess glucose as starch for later use
- Animals store extra glucose as glycogen in the liver and muscles
3. Structural Support
- Cellulose strengthens plant cell walls and helps maintain shape
- Chitin provides protection and rigidity in fungal cell walls and arthropod exoskeletons
- Acts as dietary fibre in humans, aiding digestion
🥩 2. Proteins
The building blocks of life and essential for structure and function
What are Proteins?
Proteins are essential components of all living cells and play a vital role in the structure and functioning of the human body. They are made up of amino acids containing the elements carbon, hydrogen, oxygen, nitrogen, and sometimes sulphur.
About 17% of the mature human body consists of proteins, reflecting their importance in growth, repair and everyday cellular activities. Foods such as meat, fish, eggs and cereals are rich sources of protein.
Amino Acids
Amino acids are the basic building blocks of proteins, essential for the structure and function of all living organisms.
General Structure:
Central carbon (C) bonded to:
- Amino group (NH₂)
- Carboxyl group (COOH)
- Hydrogen atom (H)
- R-group (variable side chain containing C and H) – determines the type of amino acid
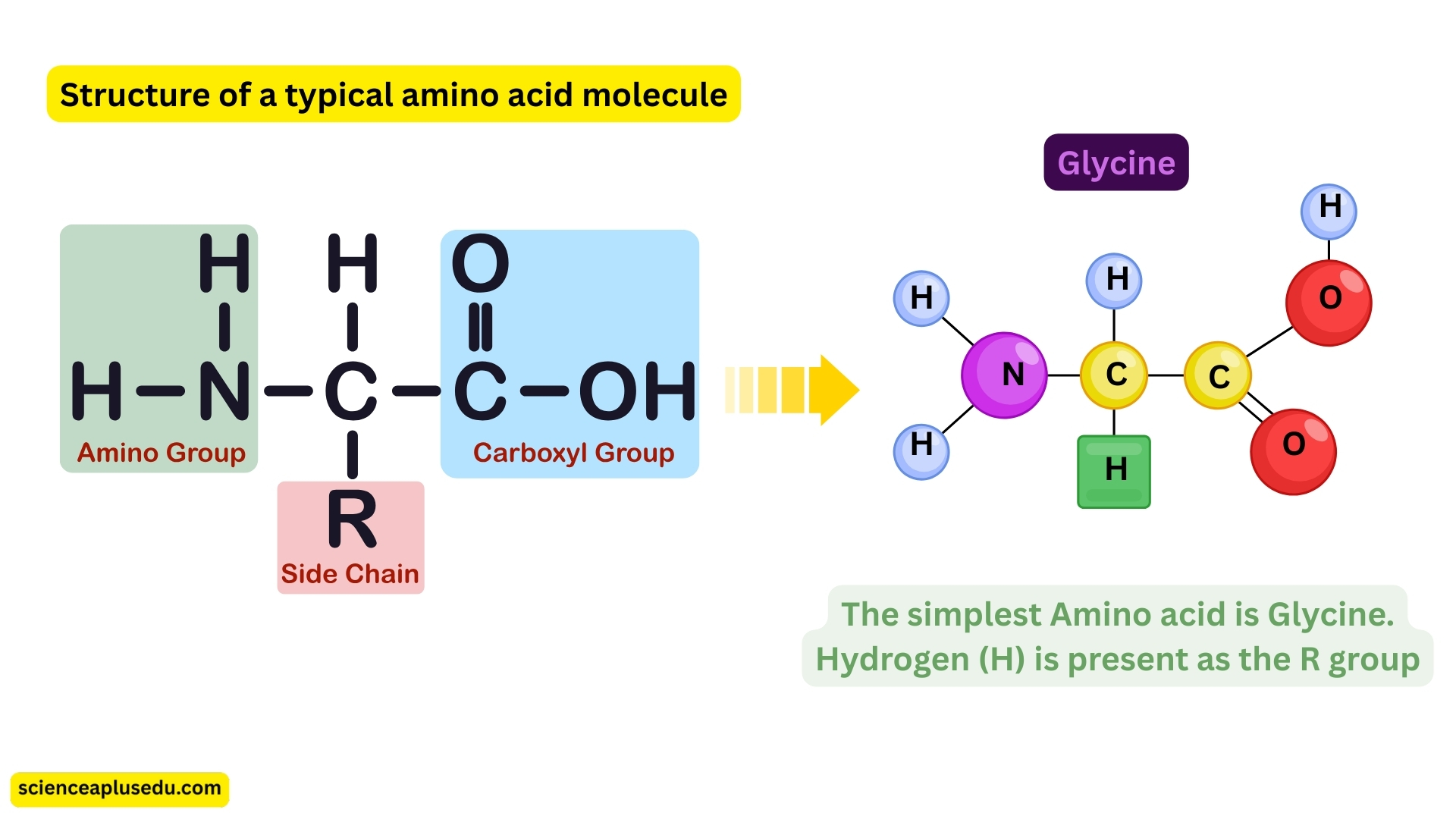
Key Points:
- There are 20 different amino acids present in all living organisms
- Proteins are formed by different sequences of these amino acids
- The simplest amino acid is glycine, where the R-group is just a hydrogen atom
- Some amino acids cannot be synthesized by the human body and must be obtained from food – called essential amino acids
Types of Amino Acids
1. Essential Amino Acids
- Cannot be synthesized by the organism and must be obtained from diet
- In humans and animals, there are 9 essential amino acids:
- Histidine, Isoleucine, Leucine, Lysine, Methionine, Phenylalanine, Threonine, Tryptophan, Valine
2. Non-Essential Amino Acids
- Can be synthesized by the organism
- Examples: Alanine, Asparagine, Aspartic acid, Glutamic acid, Serine
3. Conditional Amino Acids
- Usually non-essential, but may become essential under certain conditions such as illness or stress
- Examples: Arginine, Cysteine, Glutamine, Tyrosine, Glycine, Proline
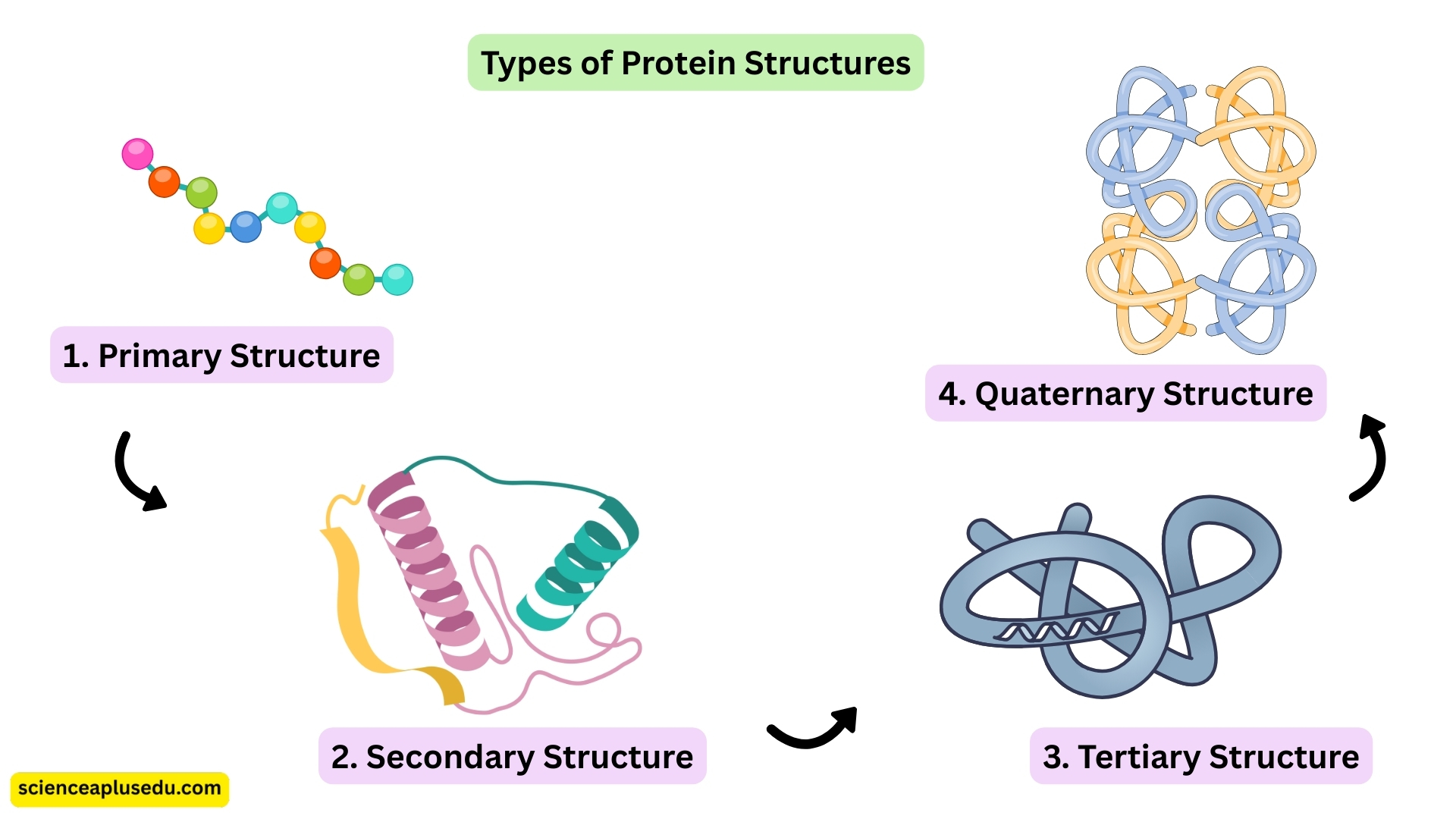
Structure of Proteins
Proteins have four levels of structure. Each level contributes to the final shape and function of the protein.
1. Primary Structure
- The exact sequence of amino acids in the polypeptide chain
- Even one amino acid change can affect the entire protein function (e.g., sickle-cell anaemia)
2. Secondary Structure
- Formed by hydrogen bonding between amino acids
- Common patterns: α-helix (spiral/coiled) and β-pleated sheet (folded, zig-zag layers)
- Adds strength and stability to the protein
3. Tertiary Structure
- The three-dimensional folding of the polypeptide chain
- Caused by interactions between R-groups (side chains) of amino acids
- Determines the protein's shape and its active site (in enzymes)
4. Quaternary Structure
- Formed when two or more polypeptide chains join together
- Example: Haemoglobin, which has four polypeptide chains working together to bind oxygen
Functions of Proteins
Enzymes
- Act as biological catalysts that speed up chemical reactions inside cells
- Each enzyme is specific to one reaction
- Example: Amylase breaks down starch into maltose
Structural Proteins
- Provide strength, support, and shape to cells and tissues
- Keratin – forms hair, nails, skin surface
- Collagen – strengthens connective tissues, bones, and skin
Transport Proteins
- Help carry substances throughout the body
- Example: Haemoglobin transports oxygen from the lungs to tissues
Hormonal, Defence, Movement & Storage Proteins
- Insulin – regulates blood glucose levels
- Antibodies – fight pathogens like bacteria and viruses
- Actin and Myosin – involved in muscle contraction
- Ferritin – stores iron in the liver
🥑 3. Lipids
Energy storage molecules and membrane components
What are Lipids?
Lipids are a group of organic compounds that include fats and oils. They are essential for storing energy, forming cell membranes, and acting as signalling molecules. Like carbohydrates, they are made up of carbon (C), hydrogen (H), and oxygen (O), but contain much less oxygen relative to carbon and hydrogen.
Key Characteristics:
- Fats are solid at room temperature, whereas oils are liquid
- Lipids are insoluble in water but soluble in organic solvents such as alcohol, ether, and chloroform
- They are a concentrated source of energy, providing about twice as much energy per gram as carbohydrates
Formation of Lipids:
Lipids are formed when fatty acids react with glycerol through a process called esterification:
Fatty acids + Glycerol → Lipid + Water
Types of Fatty Acids
1. Saturated Fatty Acids
- Contain only single bonds between carbon atoms
- Straight chains that pack closely together, making them solid at room temperature
- Common sources: butter, ghee, and animal fats
2. Unsaturated Fatty Acids
- Contain one or more double bonds between carbon atoms
- Double bonds introduce kinks, preventing tight packing, so they are liquid at room temperature
- Common sources: vegetable oils, olive oil, and fish oil
Types of Lipid Molecules
| Type of Lipid | Structure / Composition | Function | Examples |
|---|---|---|---|
| Fats (Triglycerides) | Glycerol + 3 fatty acids | Energy storage, insulation, protection | Butter, Ghee, Lard, Coconut fat |
| Oils (Triglycerides) | Glycerol + 3 fatty acids (liquid at room temp) | Energy storage, supply of essential fatty acids | Olive oil, Groundnut oil, Sunflower oil |
| Phospholipids | Glycerol + 2 fatty acids + Phosphate group | Form cell membranes, cell signaling | Phosphatidylcholine, Lecithin |
| Steroids | Four-ring carbon structure | Hormones, membrane fluidity | Cholesterol, Testosterone, Estrogen |
| Waxes | Long-chain fatty acids + alcohols | Protective coatings, waterproofing | Earwax, Beeswax, Cuticle wax |
Functions of Lipids
- Energy Source: Lipids provide more than twice the energy per gram compared to carbohydrates
- Structural Components: Phospholipids and cholesterol are critical for cell membranes
- Water Conservation: Waxes prevent water loss in plants (cutin) and animals
- Temperature Regulation: Hypodermal fat acts as thermal insulation in mammals
- Organ Protection: Fat cushions protect vital organs from mechanical injury
- Hormone Synthesis: Lipid-based hormones include Oestrogen, Testosterone, and Cortisone
🧬 4. Nucleic Acids
The genetic blueprints of life
What are Nucleic Acids?
Nucleic acids are one of the most important biomolecules in living organisms, especially in terms of genetics. They are linear polymers made up of nucleotides and are responsible for storing and transmitting genetic information.
Nucleic acids contain: Carbon (C), Hydrogen (H), Oxygen (O), Nitrogen (N), and Phosphorus (P).
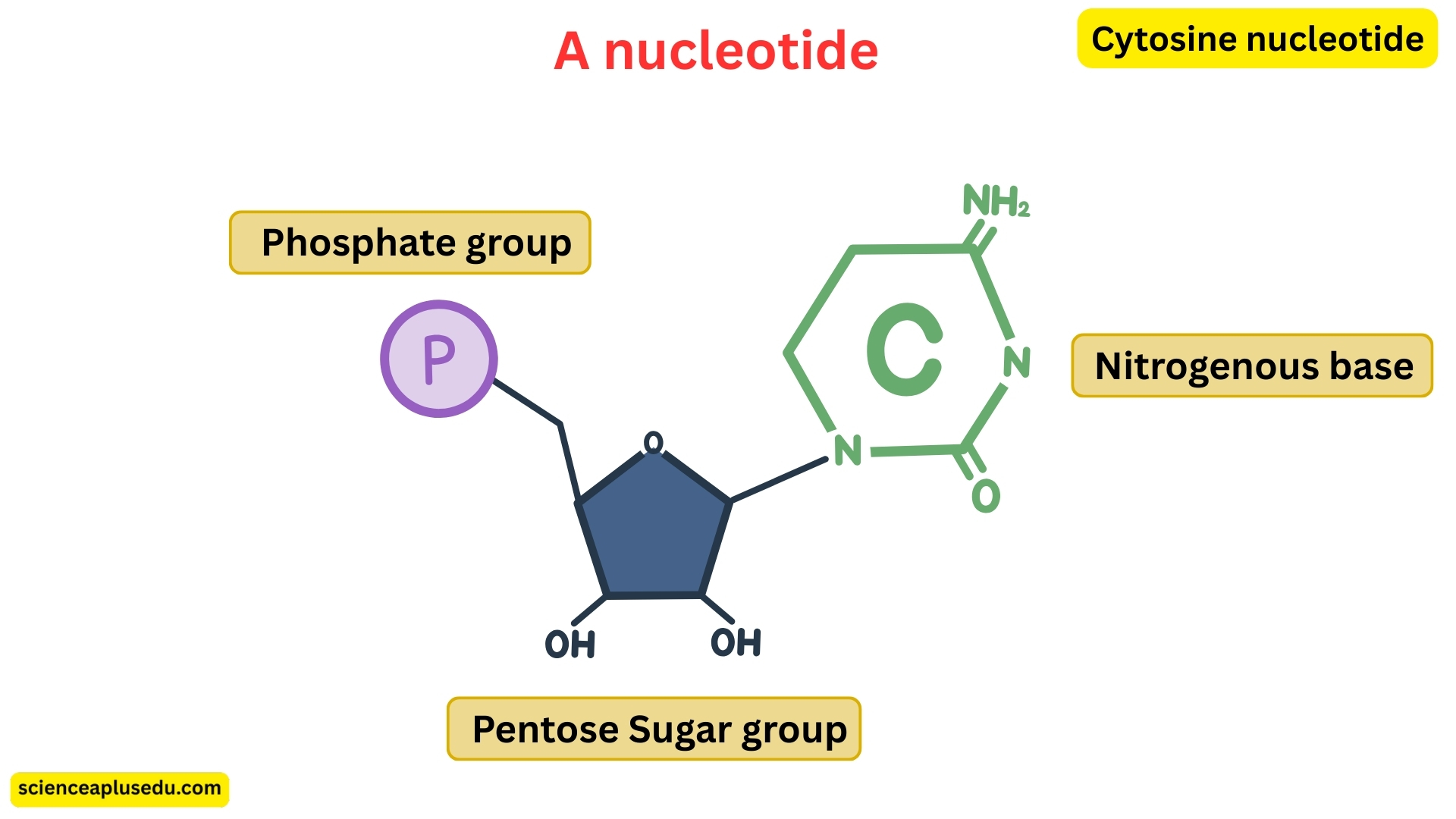
Structure of Nucleotides
Each nucleotide, the building block of nucleic acids, consists of three components:
- Nitrogenous Base – a molecule containing nitrogen that forms the "letters" of the genetic code
- Pentose Sugar – a five-carbon sugar:
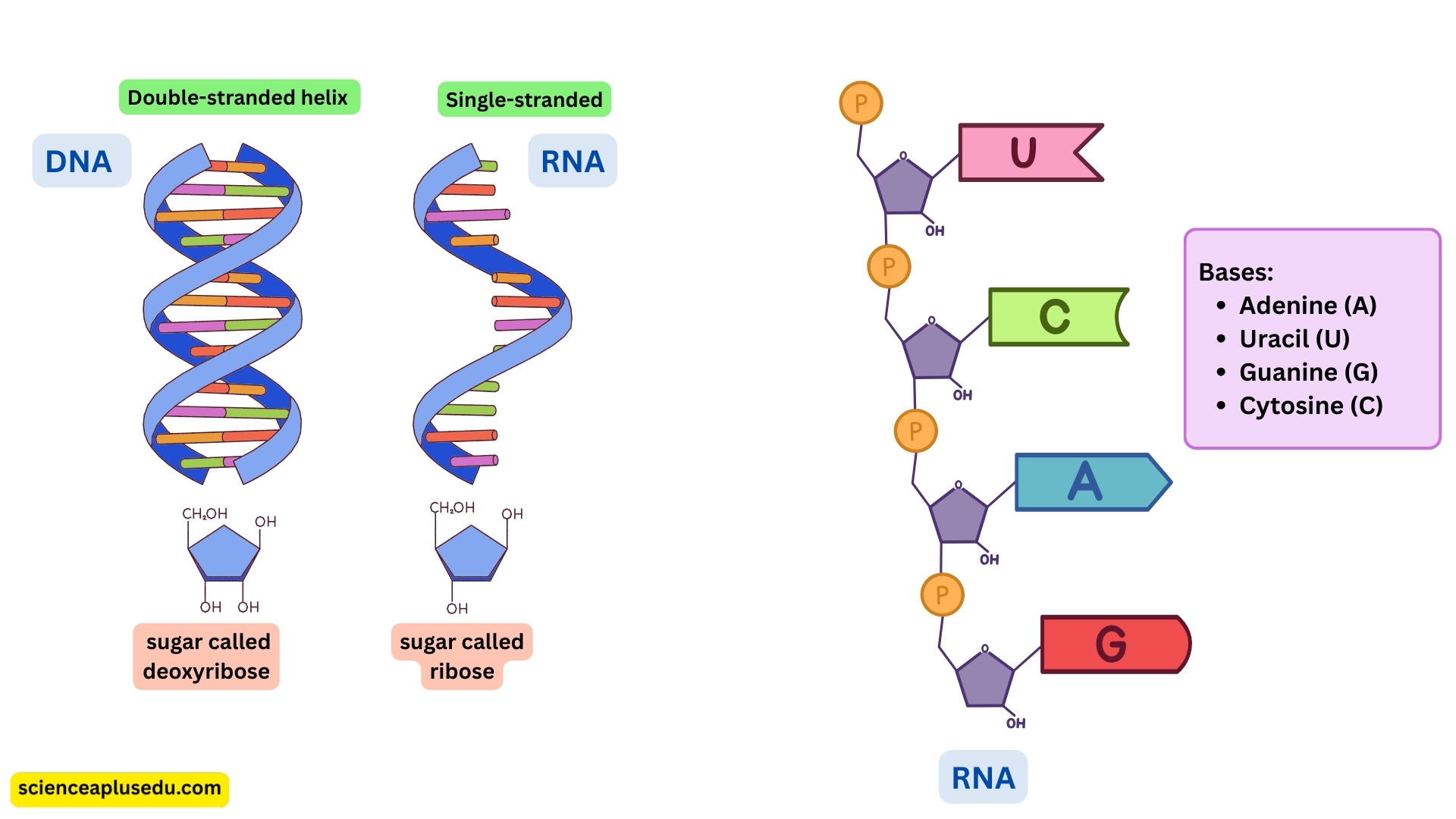
- DNA contains deoxyribose
- RNA contains ribose
- Phosphate Group – links nucleotides together, forming the backbone
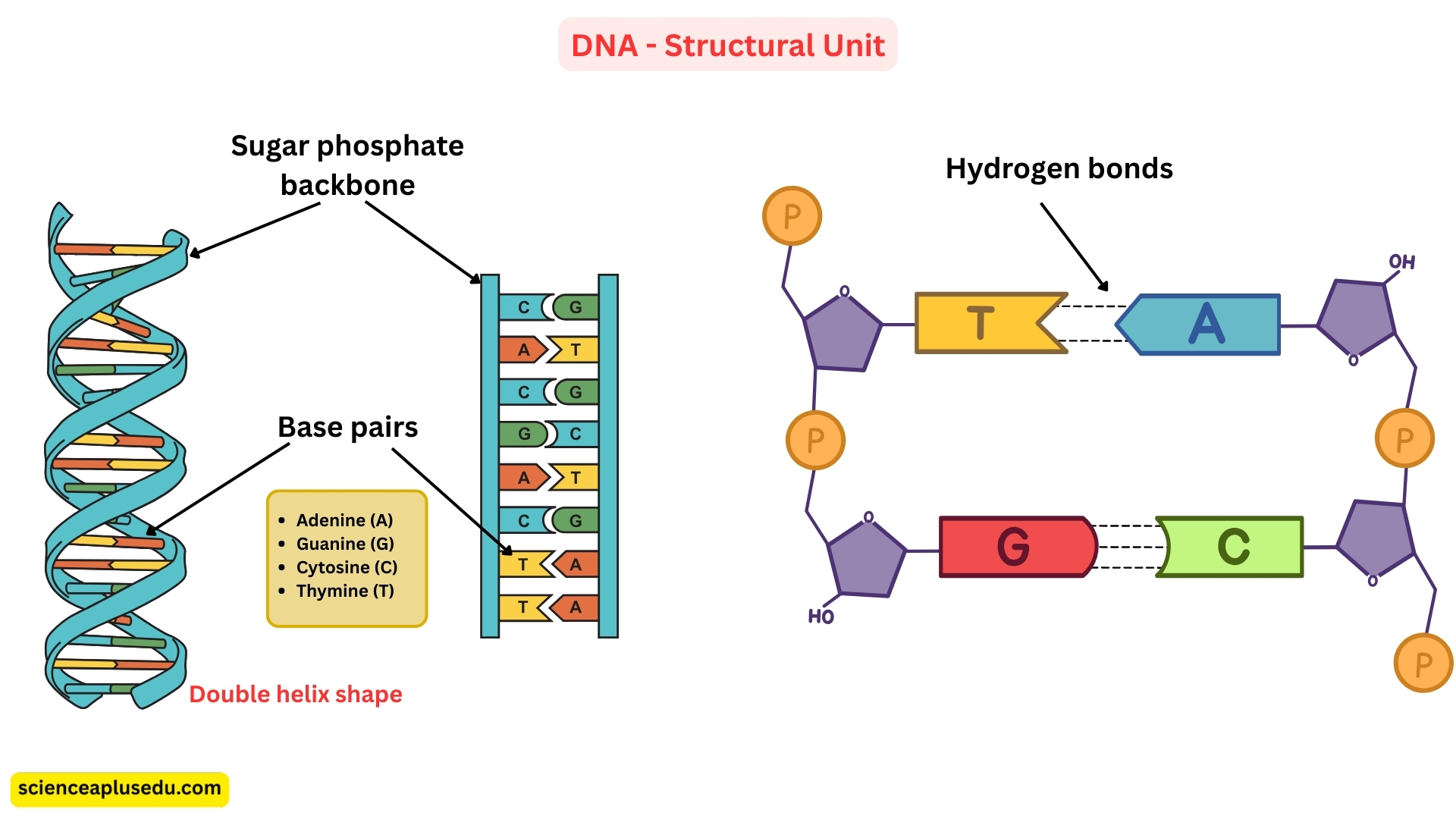
DNA (Deoxyribonucleic Acid)
Structure:
- DNA is double-stranded, forming the well-known double helix shape
- The two strands are held together by hydrogen bonds between complementary bases (A-T and G-C)
- Has a sugar-phosphate backbone
- Bases: Adenine (A), Thymine (T), Guanine (G), Cytosine (C)
Functions:
- Stores genetic information that determines inherited traits
- Transfers genetic information from one generation to the next
- Controls cellular activities by providing instructions for protein synthesis
- Supports evolution through variations and mutations
RNA (Ribonucleic Acid)
Structure:
- RNA is usually single-stranded
- Contains ribose sugar instead of deoxyribose
- Bases: Adenine (A), Uracil (U), Guanine (G), Cytosine (C) – Uracil replaces Thymine
Functions:
- Protein synthesis: RNA decodes genetic instructions from DNA and helps assemble amino acids
- Genetic material in some viruses: Some viruses use RNA instead of DNA
💧 5. Water and Inorganic Ions
Essential inorganic compounds for life
Importance of Water
Water is the most abundant inorganic compound in living organisms, making up about two-thirds (2/3) of body weight. It is an essential medium for life, as all biochemical reactions occur in aqueous environments.
Properties of Water and Their Contribution to Life
| Property of Water | Contribution to Life |
|---|---|
| Solvent | Dissolves a wide range of substances (salts, sugars, gases), allowing chemical reactions to occur in cells |
| High Specific Heat | Helps maintain stable body and environmental temperatures, protecting organisms from extreme temperature changes |
| Cohesion and Adhesion | Enables water transport in plants (capillary action) and maintains surface tension for aquatic life |
| Density and Ice Formation | Ice floats, insulating aquatic life in cold climates and preventing freezing of entire water bodies |
| Reactant in Reactions | Participates in reactions like hydrolysis and photosynthesis |
| Transport Medium | Carries nutrients, gases, and wastes in plants and animals |
| Lubricant and Cushion | Provides cushioning for organs and joints (e.g., cerebrospinal fluid, synovial fluid) |
Minerals
Minerals are inorganic nutrients essential for maintaining life processes. They make up about 7% of body weight, with calcium and phosphorus accounting for ¾ of this amount.
Classification:
- Macro elements (Macrominerals): Required in larger amounts – Calcium, Phosphorus, Potassium, Magnesium
- Trace elements (Microminerals): Required in very small amounts – Iron, Copper, Iodine, Zinc
Functions of Minerals in Human Body and Deficiency Symptoms
| Element | Functions | Deficiency Symptoms |
|---|---|---|
| Calcium | Growth of bones and teeth, blood clotting, proper function of nerves | Weakening of bones and teeth, growth disorders, osteoporosis |
| Iron | Constituent of haemoglobin, energy release in muscles | Anaemia, sleepiness, weakness |
| Iodine | Synthesis of thyroxin, growth and development | Goiter |
| Potassium | Controls ionic balance, heart and muscle activity, nerve impulses | Weakening of muscles, psychological disorders |
| Sodium | Activates enzymes, maintains osmotic pressure, nerve impulses | Respiratory disorders, cramps, nausea |
| Phosphorus | Growth of bones and teeth, constituent of nucleic acid | Weakening of bones and teeth |
Functions of Minerals in Plants and Deficiency Symptoms
| Element | Functions | Deficiency Symptoms |
|---|---|---|
| Nitrogen | As a constituent of amino acid, proteins, nucleic acid and chlorophyll | Retardation in growth, Chlorosis in mature leaves |
| Phosphorous | As a constituent of nucleic acid and ATP (Adenosine Tri Phosphate) | Retarded growth of roots, Red and purple patches on leaves |
| Potassium | Protein synthesis, Opening and closing of stomata | Chlorosis in leaves, Yellow or brown patches in leaves |
| Iron | Synthesis of chlorophyll, Synthesis of respiratory enzymes | Chlorosis in tender leaves |
| Calcium | Component of cell wall, To maintain the structure and functions of plasma membrane, For the activity of enzymes | Dying of tissues at the tips of the leaves |
| Zinc | For the activity of most enzymes, Synthesis of chlorophyll | Dead cells and tissues throughout the plant, Extra thickness in leaves |
| Sulphur | As a constituent of amino acids and proteins | Chlorosis in veins and areas between veins |
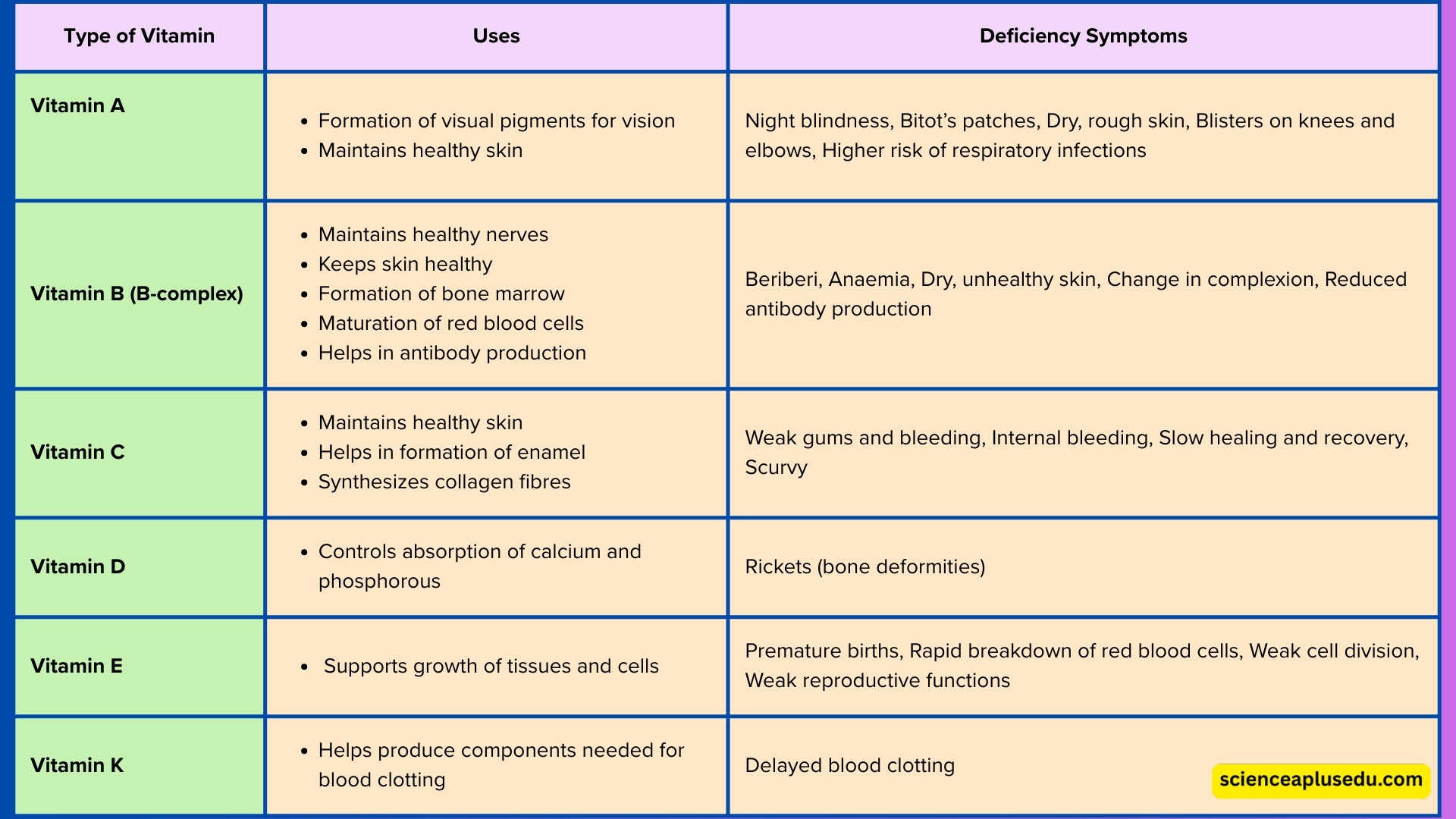
💊 6. Vitamins
Organic compounds essential for health
What are Vitamins?
Vitamins are organic compounds essential for proper body functioning. They play a key role in biochemical reactions, helping enzymes work efficiently and supporting growth, development, and overall health.
Classification by Solubility:
- Water-Soluble Vitamins: Dissolve in water and easily transported in bloodstream
- Examples: Vitamin B complex and Vitamin C
- Not stored in large amounts – need regular consumption
- Fat-Soluble Vitamins: Dissolve in fats and oils
- Examples: Vitamin A, D, E, and K
- Can be stored in fatty tissues and liver for later use